Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Supportive care - Health Professional Information [NCI]
Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Supportive care - Health Professional Information [NCI]Skip to the navigationOverviewThe American Cancer Society estimates that more than 600,000 Americans will die of cancer in 2017.[1] Anticipating the end of life (EOL) and making health care decisions about appropriate or preferred treatment or care near the EOL is intellectually challenging and emotionally distressing for patients with advanced cancer, their families and friends, oncology clinicians, and other professional caregivers. However, the adverse consequences of failing to plan for the transition to EOL care include the following: - Increased psychological distress.
- Medical treatments inconsistent with personal preferences.
- Utilization of burdensome and expensive health care resources of little therapeutic benefit.
- A more difficult bereavement.
Oncologists and patients often avoid or delay planning for the EOL until the final weeks or days of life because of many potential factors. These factors can be at the individual, family, or societal levels. Emerging evidence suggests, however, that many of the listed factors are not truly barriers and can be overcome. The purpose of this summary is to review the evidence surrounding conversations about EOL care in advanced cancer to inform providers, patients, and families about the transition to compassionate and effective EOL care. References:
-
American Cancer Society: Cancer Facts and Figures 2017. Atlanta, Ga: American Cancer Society, 2017. Available online. Last accessed May 25, 2017.
Quality of End-of-Life Care in Patients With Advanced CancerThe patient with advanced cancer, his or her family and friends, and the oncology clinicians often are faced with treatment decisions that profoundly affect the patient's quality of life (QOL). Oncology clinicians are obligated to explore, with the patient and family, the potential impact of continued disease-directed treatments or care directed at the patient's symptoms and QOL. This section summarizes the information that will allow oncology clinicians and patients with advanced cancer to create a plan of care to improve QOL at the end of life (EOL) by making informed choices about the potential harms of continued aggressive treatment and the potential benefits of palliative or hospice care. This section: - Reviews the concept of quality EOL care.
- Summarizes and evaluates the commonly cited indicators of quality EOL care.
- Explores the concept of a good death.
In addition, information about outcomes associated with cardiopulmonary resuscitation (CPR) and admission to the intensive care unit (ICU) at the EOL will allow the oncology clinician to better present options to patients with advanced cancer who are near the EOL. Quality of EOL Care Questions relevant to the goal of measuring the quality of EOL care in patients with advanced cancer include the following:[1] - Which evidence-based guidelines inform assessments of quality?
- What period defines the EOL?
- Are the quality indicators of interest accurate, readily available, and plausibly linked to desired outcomes?
- What constitutes high quality?
- Most important, is the patient's perspective given precedence?
The patient perspective Surveys and interviews of patients with life-threatening illnesses, not restricted to cancer, can contribute to the understanding of what constitutes high-quality EOL care. One group of researchers proposed that patients value five main domains of care near the EOL:[2] - Receiving adequate pain and symptom management.
- Avoiding inappropriate prolongation of dying.
- Achieving a sense of control.
- Relieving burden.
- Strengthening relationships with loved ones.
A 2011 prospective study of QOL in a cohort of patients with advanced cancer seen in outpatient medical oncology clinics provides additional insight into the patient's perspective of what contributes to a good QOL in advanced cancer.[3] The median survival of the cohort was 10 months, so the results may not reflect the situation for patients closer to death. Nonetheless, the strongest predictors of QOL were age, good performance status, and a survival time longer than 6 months. Patients who were waiting for a new treatment had worse emotional well-being. This experience suggests that QOL is related to factors such as disease progression and its complications, and to patients' goals relative to any treatment they are receiving. In addition, other researchers have reported that family caregivers often report worse scores than do patients with advanced cancer in self-assessments of the patients' QOL.[4] Indicators of EOL care quality A variety of indicators have been proposed to measure the quality of EOL care in patients with advanced cancer. Several salient criticisms of the proposed indicators include the following: - Indicators are typically measured in a period before death that is defined retrospectively from death. Clinicians cannot predict whether an intervention will be futile in preventing death; consequently, quality concerns may be exaggerated if the indicator is dependent on the time to death.
- Quality indicators may be insensitive to patient preferences. For example, a patient may prefer to receive chemotherapy close to death and forgo hospice enrollment. Conversely, the failure to deliver treatments consistent with guidelines may reflect patient refusals or medical contraindications to the recommended treatment. One study [5] demonstrated that adherence to six quality indicators in patients with lung cancer cared for in the Veterans Health Administration (VHA) was compromised by refusal (0% to 14% of the time) or medical contraindications (1% to 30% of the time), depending on the indicator.
- Administrative databases do not capture data for all patients. For example, Centers for Medicare & Medicaid Services databases capture only the Medicare population.
- Many indicators were not deliberately developed as measures of quality and may be insensitive to important outcomes.
Nonetheless, important insights into quality EOL care are provided by studying indicators over time or between different geographic regions, health systems, or subspecialties. Trends over time in indicators of EOL care quality Multiple reports are relevant to understanding trends in EOL care quality indicators over time for a variety of cancers. The following observations are supported by a 2004 analysis,[6] with additional supporting citations indicated when relevant: - Increasing numbers of patients start a new chemotherapy regimen within 30 days of death or continue to receive chemotherapy within 14 days of death.
- Increasing numbers of patients are referred to hospice; however, the length of stay in hospice remains relatively brief, supporting the concern that referrals to hospice may occur too late. For example, a 2011 study of men dying from prostate cancer demonstrated an increased utilization of hospice (approximately 32% to 60%), but there was an increase in the proportion of stays shorter than 7 days.[7]
- The rates of utilization of ICU stays have also increased. For example, one study [8] reported that for patients dying from pancreatic cancer, admissions to ICUs increased in two different time periods: from 1992 to 1994, ICU admissions increased from 15.5% to 19.6%; and from 2004 to 2006, ICU admissions increased from 8.1% to 16.4%.
- Rates of do-not-resuscitate (DNR) orders have increased but remain close to death: A 2008 study of the rate and timing of DNR orders at a major cancer center between the years 2000 and 2005 demonstrated that the rates of DNR at the time of death increased from 83% to 86% for in-hospital deaths and from 28% to 52% for out-of-hospital deaths.[9] However, for inpatient deaths, the median time between signing the DNR and death was 0 days; for outpatient deaths, it was 30 days. This suggests that communication about resuscitation preferences is delayed. This delay may negatively affect patient preparation for the EOL.
Regional variations in indicators of EOL care quality Regional variations in rates of utilization of health care resources near the EOL are of interest because the differences are rarely associated with improved outcomes. While initial findings focused on differences between geographic regions of the United States, subsequent studies have demonstrated potentially meaningful differences between or within health systems.[10] A brief summary of some notable variations follows. - Compared with men enrolled in a fee-for-service Medicare product, older men with advanced cancer who received treatment through the VHA were less likely to receive chemotherapy within 14 days of death (4.6% vs. 7.5%), less likely to spend time in an ICU within 30 days of death (12.5% vs. 19.7%), and less likely to visit the emergency department more than once (13.1% vs. 14.7%).[11]
- An analysis of data by the Dartmouth Institute for Health Policy & Clinical Practice about the care of Medicare patients with poor prognoses demonstrated significant regional variations in EOL care.[12] The principal findings include the following:
- Variations in the rate of hospitalizations, ICU stays, and aggressive interventions such as CPR and mechanical ventilation.
- Variations in the use of chemotherapy in the last 14 days of life.
- Variations in the use of hospice measured as rates of referral or lengths of stay.
In the opinion of the authors, the observed regional variations were too large to be accounted for by racial or ethnic preferences or illness levels. Factors correlated with the aggressiveness of EOL care include the availability of resources such as the supply of ICU beds or imaging equipment; the number of doctors involved in each patient's care; and the treatment setting itself.[12]
Factors associated with variations in EOL care Availability of medical specialists, numbers of hospital beds, physician, and health system characteristics are well-established factors associated with increased expenditures in the final 6 months of life. A 2011 study [13] utilized data from decedents enrolled in the Health and Retirement Study, Medicare, and the Dartmouth Atlas to identify patient-level factors that may contribute to regional variations. Functional status decline, Hispanic ethnicity, black race, and chronic diseases such as diabetes were associated with higher expenditures, even after controlling for regional characteristics. The patient-level factors accounted for only 10% of variations. EOL practice patterns and number of hospital beds per capita were also associated with higher expenditures. Advance care planning did not influence expenditures.[13] A potential explanation for regional variations in EOL care is regional variations in patient preferences. The evidence, however, is conflicting. - One study surveyed 2,515 Medicare beneficiaries about their general preferences for medical care in the event of a serious life-limiting illness with a life expectancy of less than 1 year.[14] The preferences were correlated with EOL spending by hospital referral region. There were no differences between preferences for palliation or life prolongation by region. Thus, it is unlikely that regional variations in preferences account for observed variation in EOL spending.
- Conversely, a secondary analysis of the potential influence of treatment site on the Coping with Cancer study outcomes demonstrated that the effect of treatment site partly resulted from differences in patient preferences.[15]
The Good Death The health care provider perspective The concept of a good death is a controversial but potentially useful construct for the oncology clinician to more clearly formulate the goals of timely, compassionate, and effective EOL care. In a 2003 British Medical Journal article about caring for the dying patient, the authors proposed that there were sufficient evidence-based guidelines to facilitate a good death; a commentary that accompanied the article stated, "Nor can professional education convey adequately just how important it is for individuals, both at the time and afterwards, to go through the death of someone they love feeling that they are experiencing a ‘good death.'"[16] Several salient points were made in subsequent letters to the editor: - The mental health of the patient and family are essential concerns in providing a good death.
- Health care provider beliefs and attitudes may diminish the chances for a good death if they interfere with adequate pain or symptom control.
- Health care providers may risk medical paternalism if they are not respectful of a patient's perspective and insist on EOL care or refuse to provide comfort measures that may shorten life.
- Religious faith and spiritual beliefs are important for many people near the EOL.
- A good death requires turning one's attention away from prolonging life.
The patient perspective A landmark study of patients, families, and health care providers [17] surveyed seriously ill patients, bereaved caregivers, physicians, and other health care providers about what matters at the EOL. Respondents scored the importance of 44 attributes identified in previous interviews and focus groups in which participants were asked to define a good death. There was broad agreement about control of pain and other symptoms, communication, and preparation for death. However, certain attributes such as not being a burden to one's family or society, or being at peace with God, were ranked as important by patients but less so by physicians. A population-based study in the Netherlands demonstrated a correlation between the respondents' positive attitudes toward the concept of a good death and preferences that could potentially influence EOL decision making.[18] Patients with advanced cancer may desire opportunities to prepare for the EOL. A survey of 469 patients who participated in a cluster-randomized trial of early palliative care [19,20] reported that better preparation was associated with higher scores related to the quality of communication with clinicians, older age, living alone, fewer symptoms, and spiritual well-being. Interestingly, 31% of respondents were worried about their family's future coping. The results highlight the importance of offering patients an opportunity to discuss preparation for the EOL early and that patients who favorably rate the quality of communication with their physicians feel more prepared. The caregiver perspective In one study,[21] formal and informal caregivers of 396 patients who died from advanced cancer were interviewed about the decedent's QOL in the last week of life. The QOL was correlated with a variety of factors obtained via baseline interviews of decedents at the time of enrollment into the study and survey measures of coping styles, religious coping, religiousness/spirituality, and EOL preferences. Most variances in the QOL at EOL were unexplained. However, factors negatively correlated with QOL included the following: - Intensive care in the last week.
- Death in the hospital.
- Feeding tube in the final week.
- Chemotherapy in the final week of life.
Conversely, factors correlated with a higher QOL included the following: - Prayer or meditation.
- Visits with chaplains.
- The perception that the physician was respectful, open to questions, and trustworthy.
Outcomes After Potentially Life-Prolonging Interventions CPR CPR was initially developed to restore circulation in patients with predominantly cardiac insults. The outcomes after inpatient CPR in older adults have not improved significantly, in part because of the use of CPR in patients with comorbid life-threatening illnesses. Addressing the limited benefit of CPR during the transition to EOL care is made more difficult because in the United States, patients will undergo CPR unless there is an established DNR order in the medical chart to countermand the procedure. The following is a summary of the evidence that demonstrates the very limited value of CPR in patients with advanced cancer: - A retrospective chart review of 41 patients who underwent CPR for an out-of-hospital cardiac arrest found that only 18 (43%) survived to be admitted to an ICU, and only 9 patients survived to be discharged (2 to home; 7 to a facility).[22] The authors noted that documentation of the advanced stage of disease and poor prognosis were frequently mentioned in the medical records.
- A meta-analysis of survival after in-hospital CPR found that survival to discharge was 6.2% overall.[23] Patients with advanced-stage disease, with hematological malignancy, and who underwent CPR in the ICU were less likely to survive to be discharged.
- Researchers have described the extremely poor outcomes of patients who were resuscitated in the medical ICU.[24,25] Only 7 of 406 patients resuscitated (2%) survived to be discharged. The remainder died at the time of the arrest (63%) or died within a mean of 4 days (26%).
Admission to an ICU In addition to CPR, patients may require mechanical ventilation or admission to ICUs. Outcomes are poor for patients with advanced cancer. One study reported that the median survival of 212 patients with advanced cancer (who were referred to a phase I trial) was 3.2 weeks.[26] Patients who required CPR had a median survival of 1 day. The underlying diagnosis, however, may be a critical variable in predicting patient outcome. Patients with hematologic malignancies may do better than patients with solid tumors. For example, one study reported that patients with newly diagnosed hematologic malignancies had a 60.7% chance of surviving to be discharged and a 1-year survival rate of 43.3%.[27] Multivariate analysis demonstrated that a remission status and early admission to the ICU were favorable risk factors. References:
-
Kendall M, Harris F, Boyd K, et al.: Key challenges and ways forward in researching the "good death": qualitative in-depth interview and focus group study. BMJ 334 (7592): 521, 2007.
-
Singer PA, Martin DK, Kelner M: Quality end-of-life care: patients' perspectives. JAMA 281 (2): 163-8, 1999.
-
Zimmermann C, Burman D, Swami N, et al.: Determinants of quality of life in patients with advanced cancer. Support Care Cancer 19 (5): 621-9, 2011.
-
Jones JM, McPherson CJ, Zimmermann C, et al.: Assessing agreement between terminally ill cancer patients' reports of their quality of life and family caregiver and palliative care physician proxy ratings. J Pain Symptom Manage 42 (3): 354-65, 2011.
-
Ryoo JJ, Ordin DL, Antonio AL, et al.: Patient preference and contraindications in measuring quality of care: what do administrative data miss? J Clin Oncol 31 (21): 2716-23, 2013.
-
Earle CC, Neville BA, Landrum MB, et al.: Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22 (2): 315-21, 2004.
-
Bergman J, Saigal CS, Lorenz KA, et al.: Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med 171 (3): 204-10, 2011.
-
Sheffield KM, Boyd CA, Benarroch-Gampel J, et al.: End-of-life care in Medicare beneficiaries dying with pancreatic cancer. Cancer 117 (21): 5003-12, 2011.
-
Levin TT, Li Y, Weiner JS, et al.: How do-not-resuscitate orders are utilized in cancer patients: timing relative to death and communication-training implications. Palliat Support Care 6 (4): 341-8, 2008.
-
Newhouse JP, Garber AM: Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA 310 (12): 1227-8, 2013.
-
Keating NL, Landrum MB, Lamont EB, et al.: End-of-life care for older cancer patients in the Veterans Health Administration versus the private sector. Cancer 116 (15): 3732-9, 2010.
-
Goodman DC, Morden NE, Chang CH, et al.: Trends in Cancer Care Near the End of Life: A Dartmouth Atlas of Health Care Brief. Lebanon, NH: Dartmouth Institute for Health Policy & Clinical Practice, 2013. Available online. Last accessed February 27, 2017.
-
Kelley AS, Ettner SL, Morrison RS, et al.: Determinants of medical expenditures in the last 6 months of life. Ann Intern Med 154 (4): 235-42, 2011.
-
Barnato AE, Herndon MB, Anthony DL, et al.: Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care 45 (5): 386-93, 2007.
-
Wright AA, Mack JW, Kritek PA, et al.: Influence of patients' preferences and treatment site on cancer patients' end-of-life care. Cancer 116 (19): 4656-63, 2010.
-
Ellershaw J, Ward C: Care of the dying patient: the last hours or days of life. BMJ 326 (7379): 30-4, 2003.
-
Steinhauser KE, Christakis NA, Clipp EC, et al.: Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 284 (19): 2476-82, 2000.
-
Rietjens JA, van der Heide A, Onwuteaka-Philipsen BD, et al.: Preferences of the Dutch general public for a good death and associations with attitudes towards end-of-life decision-making. Palliat Med 20 (7): 685-92, 2006.
-
Zimmermann C, Swami N, Krzyzanowska M, et al.: Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 383 (9930): 1721-30, 2014.
-
Wentlandt K, Burman D, Swami N, et al.: Preparation for the end of life in patients with advanced cancer and association with communication with professional caregivers. Psychooncology 21 (8): 868-76, 2012.
-
Zhang B, Nilsson ME, Prigerson HG: Factors important to patients' quality of life at the end of life. Arch Intern Med 172 (15): 1133-42, 2012.
-
Hwang JP, Patlan J, de Achaval S, et al.: Survival in cancer patients after out-of-hospital cardiac arrest. Support Care Cancer 18 (1): 51-5, 2010.
-
Reisfield GM, Wallace SK, Munsell MF, et al.: Survival in cancer patients undergoing in-hospital cardiopulmonary resuscitation: a meta-analysis. Resuscitation 71 (2): 152-60, 2006.
-
Ewer MS, Kish SK, Martin CG, et al.: Characteristics of cardiac arrest in cancer patients as a predictor of survival after cardiopulmonary resuscitation. Cancer 92 (7): 1905-12, 2001.
-
Wallace S, Ewer MS, Price KJ, et al.: Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer 10 (5): 425-9, 2002.
-
Fu S, Hong DS, Naing A, et al.: Outcome analyses after the first admission to an intensive care unit in patients with advanced cancer referred to a phase I clinical trials program. J Clin Oncol 29 (26): 3547-52, 2011.
-
Azoulay E, Mokart D, Pène F, et al.: Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 31 (22): 2810-8, 2013.
Factors That Influence End-of-Life Care Decisions and OutcomesOverview One interpretation of the evidence summarized in the section on Quality of End-of-Life Care in Patients With Advanced Cancer is that patients with advanced cancer too often receive burdensome and potentially harmful treatments without much chance of benefit and to the detriment of receiving purposeful end-of-life (EOL) care. Studies of patients with advanced cancer have identified factors that can influence EOL health care decisions and outcomes. This section provides the oncology clinician with insights about potentially influential factors that may lead to more effective interactions with the patient in planning the transition to EOL care. Several notes of caution about the cited studies, however, are highlighted in the following: - The cross-sectional design of most studies prevents conclusions about causality. For example, studies that demonstrate a correlation between a patient's optimistic predictions of prognosis and treatment decisions may be confounded by the tendency for the patient who chooses a specific treatment to be optimistic about the outcome, rather than the patient's optimism being the primary reason for treatment.
- Definitions of advanced cancer, median survival times of enrolled subjects, and study methodologies may all be relevantly different across studies, and uncontrolled or unrecognized confounders may skew reported correlations.
- Many of the potentially relevant factors were treated as primary or secondary outcomes or as only one variable in multivariate analysis. Thus, the relationships among patient-oncologist communication, health care decision making near the EOL, and outcomes are not always firmly established. Nonetheless, the evidence from multiple sources demonstrates plausible and compelling links.
- Factors unrelated to communication or decision making may influence the health care decisions of patients with advanced cancer near the EOL.
Cited Studies Three very large, comprehensive studies provide a rich source of information for characterizing the relationships among markers of quality communication, decision making, health care decisions, and outcomes in patients with advanced cancer. These studies are described here, and results are integrated into subsequent sections. - The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT): This multisite (Ohio, North Carolina, Massachusetts, Wisconsin, and California) two-phase study evaluated an intervention to improve physician understanding of patient preferences, documentation and timing of do-not-resuscitate (DNR) orders, pain, time in intensive care, being comatose or receiving mechanical ventilation before death, and the use of hospital resources.[1] The first phase was a prospective descriptive study to confirm barriers and gaps in patient-physician communication; the second phase was a cluster randomized trial to test an intervention that targeted the identified barriers. A total of 4,301 patients were enrolled in phase I, and 4,804 were enrolled in phase II. The intervention focused on providing reliable information about prognosis, documenting patient and family preferences, and using a skilled nurse to educate patients and families and facilitate communication.
Phase I of SUPPORT confirmed there were significant shortcomings in patient-physician communication.[2,3] Phase II demonstrated that the nurse-led intervention was not successful in increasing discussions about CPR preferences or concordance between patients and physicians about patients' CPR preferences; or decreasing the number of days spent in the intensive care unit (ICU), frequency of mechanical ventilation, or level of pain. - The Coping with Cancer (CwC) study: This prospective, longitudinal, multisite study of terminally ill cancer patients and their informal caregivers examined how psychosocial factors influence patient care and caregiver bereavement.[4] CwC enrolled 718 patients and their caregivers between September 2002 and August 2008. Key eligibility criteria included a diagnosis of advanced cancer (distant metastases and disease refractory to first-line chemotherapy) and presence of an informal caregiver. The median overall survival of enrolled patients was 4.5 months.
- The Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium: This study enrolled more than 5,000 people diagnosed with lung or colorectal cancer between 2003 and 2005 who were identified within weeks of their diagnoses by a rapid-ascertainment method.[5,6] Patients lived in one of five geographic areas (Northern California; Los Angeles County, California; North Carolina; Iowa; or Alabama) or received care through a designated health maintenance organization or selected Veterans Health Administration hospital. Patients (or surrogates, if patients had died or were unavailable) were interviewed via computer-assisted surveys 4 to 7 months after diagnosis. The medical records of consenting adults were also abstracted.
Patient Demographics The goal of planning the transition to EOL care in a deliberate and thoughtful manner is to increase the likelihood that a person with advanced cancer will receive high-quality EOL care consistent with their informed preferences. A variety of patient characteristics influence the interaction with the oncology clinician and the patient's decisions or outcomes. The following section highlights representative results of studies of patient demographics and other factors. Age Cancer is a disease of older adults.[7] It is important to remember, however, that there are probably differences in different cohorts of older adults. - SUPPORT demonstrated a correlation between increasing age and a decreased desire for life-prolonging treatment. Older patients were less likely to receive aggressive treatments.[1]
- Seventy-three patients aged 70 to 89 years with metastatic colon cancer were interviewed about treatment decision making. Fewer than half (44%) of the patients wanted information about expected survival when they made decisions about treatment.[8]
- Older women are more likely than younger women to receive palliative care near the EOL. One study reported that 69.6% of women who died from metastatic breast cancer between 1992 and 1998 in the province of Quebec did so in an acute-care hospital.[9] While most women (75%) had some indicators of a palliative care-oriented model (as extracted from information contained in two administrative databases), women younger than 50 years were less likely to receive palliative care than were women older than 70 years (odds ratio [OR], 1.85).
- One study analyzed the relationships between age, treatment preferences, and treatment received in a cohort of 396 deceased patients who were enrolled in CwC.[10] Patients older than 65 years and patients aged 45 to 64 years were less likely than younger patients to prefer and receive life-prolonging treatments. However, older patients who preferred life-prolonging therapies were less likely than younger patients to receive such treatments.
Gender - In one study,[8] older men with metastatic colorectal cancer were more likely than women to desire prognostic information (56% vs. 29%; P < .05)
- Another study interviewed patients with advanced cancer before and after a visit with an oncology clinician to discuss scan results. Women were more likely to recognize the cancer as incurable and to accurately identify the stage of the cancer. Women were also more likely to report that discussions about life expectancy occurred.[11]
Race - A secondary analysis of CwC demonstrated that black patients were more likely to receive intensive treatments near the EOL. The frequency of high-intensity treatments near the EOL did not correlate with stated preferences and, unlike the case with white patients, EOL discussions did not decrease the likelihood of intensive treatments.[12] This effect of race persisted even if a DNR order was in place at the time of death.[13]
- Another study analyzed interviews with terminally ill white and African American patients to determine whether the patient-reported quality of relationships with physicians correlated with advance care planning (ACP) and preferences for life-sustaining treatment (LST).[14] African American patients reported lower-quality relationships, but these lower ratings did not explain the lower rates of ACP or higher rates of preferences for LST by African Americans.
- Patients of African American or Asian descent are less likely to enroll in hospice and more likely to receive aggressive treatments, including hospitalizations and ICU admissions, than are white patients.[15,16]
Socioeconomic status - Patients with Medicaid are less likely to receive hospice care than are patients insured through Medicare, and are more likely to die in acute-care facilities.[17] Conversely, in a Medicaid population, black patients are more likely to show evidence of EOL discussions.[18]
- Medicare beneficiaries enrolled in a managed care program were more likely to enroll in hospice and to enroll for longer periods of time.[19]
Patient Understanding of Prognosis Multiple studies have demonstrated correlations between patients' understanding of their prognoses and health care decisions, medical outcomes, or psychological adjustment near the EOL. However, differences in study methodology, patient populations, measures of prognostic understanding, and the endpoint studied as the primary outcome of interest preclude definitive conclusions about the relevance of the correlations. Furthermore, causality can only be inferred, given the cross-sectional nature of most studies. Nonetheless, a summary of the published data organized by the measure of prognostic understanding may provide insight into the decision-making processes of patients with advanced cancer. - Patients often provide overly optimistic estimates of the likelihood of survival beyond 6 months. One group of researchers analyzed the prognostic understanding (measured as an estimate of surviving beyond 6 months) of 917 adults with metastatic colorectal or lung cancer who were enrolled in SUPPORT.[3] Patients who estimated a 90% or higher chance of surviving 6 months were more likely to prefer life-extending therapy and were more likely to experience a readmission, attempted cardiopulmonary resuscitation, or death while on a mechanical ventilator.
- Patients frequently fail to correctly report the goal of anticancer treatment. One group of researchers reported that at baseline, 30.4% of a cohort of 181 patients with advanced cancer believed the treatment was curative; 20% reported they did not know.[20] Subjects who were not married, lived in rural areas, died within 6 months, or were receiving chemotherapy were more likely to report the goal of treatment as curative. At a 12-week assessment, fewer patients reported cure as a goal, but the difference was not significant. The results of this study are consistent with those of other studies reported between 1984 [21] and the present.
- Patients are often unaware of the terminal nature of the diagnosis of advanced cancer. However, patients who are aware of their terminal diagnosis have a higher quality of life (QOL) [22] and are more likely to receive care consistent with their preferences.[23]
Patient Preferences Respect for patient preferences is essential to high-quality cancer care and to protecting patient autonomy. Patients with advanced cancer who had an opportunity to discuss their EOL preferences were more likely to receive care consistent with their preferences.[23] However, evidence suggests oncology clinicians often do not elicit or clarify patient preferences and, ultimately, fail to provide care consistent with their preferences. For example, a 2011 study [24] of 128 patients with newly diagnosed advanced-stage lung cancer found that: - 88.2% wanted to be informed about life expectancy (52.7% said they were informed).
- 63.5% wanted to be informed about palliative care (25% said they were informed).
- 56.8% wanted to be informed about EOL decisions (31% said they were informed).
- None of the patients recalled being asked about their information preferences.
The final observation highlights the fundamental question of how oncology clinicians should elicit patient preferences. While direct questioning may seem the most straightforward approach, a study of two single-item preference measures demonstrated that the decision-making preferences of patients appear to differ on the basis of what measure was utilized.[25] The optimal way to elicit preferences, which would reduce the likelihood of failing to respect patients' wishes, is not certain. Patients with advanced cancer have several preferences of potential significance to planning the transition to EOL care, including: - Timing and manner of prognostic information disclosure.
- Decision-making role.
- Palliative chemotherapy rather than palliative care without chemotherapy.
- QOL or length of life.
A discussion about preferences is complicated: preferences may be narrowly construed or may reflect the fundamental values of an individual. In a study of 337 older patients' attitudes about using advance directives to manage EOL care,[26] 85% of respondents believed it was definitely (55%) or somewhat (30%) necessary to record their wishes in advance directives. However, most (80%) preferred either general value and goal statements or both precise and general directions. There was a strong preference for discussion among surrogate decision makers on behalf of the incapacitated person.[26] Preference for information about prognosis Patients with life-limiting illnesses desire information about prognosis,[27] believe that such information may be provided without compromising hope,[28] and prefer that oncologists inquire about their preferences for such information.[29] Younger patient age, female sex, and a shorter life expectancy as perceived by the patient correlate with increased information needs.[8] Preference for decision-making role A variety of measures have been developed to assess patients' preferences for a decision-making role. The Control Preference Scale [30] asks patients to select one of five statements that best reflects their approaches; results are coded active ("I prefer to make the final selection"), collaborative ("I prefer that my doctor and I share responsibility"), or passive ("I prefer that my doctor makes the final decision"). The available information suggests that preferences vary widely, although how much the variation is caused by differences between disease and types of patients or reflects methodological differences is not known. - Patients aged 70 to 89 years with metastatic colorectal cancer were interviewed about their preferred role in decision making.[8] Fifty-two percent preferred a passive role. Preference for a passive role was more common among patients who were older or female or had a poorer performance status or newly diagnosed metastatic disease.
- In a separate study, a similar proportion (47%) of women aged 31 to 83 years with metastatic breast cancer reported taking a passive role in decision making about palliative chemotherapy. Women who were facing a decision about second-line chemotherapy reported a slightly higher rate of active participation (43% vs. 33%; P = .06).[31]
- A study of patients with advanced cancer in a palliative care clinic demonstrated that 63% of patients preferred an active role in decision making.[32]
Preference for palliative chemotherapy Several studies have identified a high prevalence of patient preference for chemotherapy before consultation with a medical oncologist.[33] For example, a group of Dutch researchers assessed the decision-making process of 140 patients with metastatic cancer for whom palliative chemotherapy or best supportive care were reasonable options.[34] Before the consultation, 68% of patients expressed a preference for chemotherapy. Seventy-eight percent of patients eventually chose chemotherapy. The only patient characteristic associated with preference for treatment was younger age. The strongest predictor of treatment choice was the preconsultation preference. The preference for chemotherapy may relate in part to the observations that patients are not fully informed or they reject the information or reinterpret it to fit their perspectives. In addition, patients may value survival more and QOL less than oncology clinicians anticipate.[35] Preference for QOL or length of life As discussed in the introductory paragraph to the Patient Preferences section of this summary, patient preferences may be narrowly or broadly construed. Preferences that are foundational to more specific preferences might be better considered as patient values.[36] In addition to the preference being measured, the methods of measuring preferences vary significantly. The interested reader may consult a 2012 review [37] for a discussion of the different instruments. The following paragraphs highlight some notable observations. - Multivariate analysis of one study [38] demonstrated that advanced cancer patients' preferences for chemotherapy were explained by higher values assigned to length of life and lower values for QOL. The last variable was measured by the Quality-Quantity Questionnaire (QQQ), which categorizes patients as favoring QOL or length of life, or having no preference.[39] The patients' preconsultation preferences were most strongly explained by striving for length of life rather than QOL. Given the lack of demonstrable survival benefit of chemotherapy in advanced cancer, the authors speculated that patients may not receive accurate information in the consultation that clearly delineates the goal of chemotherapy.
- Another study [40] interviewed 125 outpatients with cancer (all stages) about their attitudes toward treatment. Patients also completed the QQQ. The investigators found that patients who were older, tired, or more negative valued QOL more than others. Patients who were within 6 months of diagnosis rated length of life as more important. It should be noted, however, that patients did not rank the importance of length relative to quality in this study. There was a correlation between striving for QOL and appreciation for ACP.
- A third study enrolled only patients with advanced cancer (459 respondents) and asked patients to rate the relative value of QOL or length of life.[41] Fifty-five percent valued QOL and length of life equally; 27% preferred QOL; and 18% preferred length of life. A preference for QOL correlated with older age, male gender, and increased levels of education. Patients with a preference for length of life also preferred less pessimistic communication from oncologists.
Patient Goals of Care Discussions about goals of care with advanced-cancer patients are considered by many to be a critical component of planning the transition to EOL care. However, the definitions of goals of care vary significantly in the relevant literature. Before discussions about the relevant observations related to goals of care in patients with advanced cancer (and other life-limiting illnesses) take place, it is important to consider whether a distinction should be drawn between goals of treatment and goals of care. - Some investigators have used the phrase goals of care to identify the goals of disease-directed treatments. Perhaps it would be clearer if such goals were labeled goals of treatment.
- Viewed another way, goals of care are distinct from goals related to treatment of disease with remittive or curative intent; these goals reflect the interests of patients and families after they have accepted that disease-directed treatments will not accomplish their intended goals and they seek "the profound human needs for meaning, comfort, and direction."[42] From that perspective, goals of care are similar to the attributes that define a good death, as discussed earlier in this summary.
Another perspective is that there is a continuum of goals and that the purpose of conversations about goals of care is to help patients identify the various alternatives for achieving their goals. A structured literature review and a qualitative analysis of palliative care consultations about goals of care support this perspective, as summarized below: - One study analyzed relevant publications to establish a categorization of relevant goals of care for patients with life-limiting illnesses.[42] The authors proposed six comprehensive goals: be cured; live longer; improve or maintain function, QOL, and independence; be comfortable; achieve life goals; and provide support for family or caregiver.
- Another study reported a qualitative analysis of prognostic communication in palliative care consultations.[43] The investigators noted that palliative care physicians used a tactic of excluding certain goals of care as a means to encourage patients to think about alternative goals. This suggests that treatment-related goals are often replaced by more personal goals once disease-directed treatment is not advisable.
At present, there are no data on the positive or negative influence of discussions about goals of care on the EOL outcomes of patients with advanced cancer. Religious and Spiritual Beliefs and Values of Patients Patient religiosity and the provision of spiritual care consistent with a patient's preference have been correlated with EOL outcomes. A series of reports from CwC [44,45,46] found that approximately half of patients with serious or life-threatening illnesses indicated that religious or spiritual beliefs were important to them, as measured by higher scores on the Positive Religious Coping Scales subscale of the RCOPE, a measure of religious coping.[47] RCOPE scores were significantly higher among African Americans and Hispanics. In analyses adjusted for demographic differences, higher levels of positive religious coping were found to be significantly related to the receipt of mechanical ventilation, compared with low levels of religious coping (11.3% vs. 3.6%) and intensive life-prolonging care during the last week of life (13.6% vs. 4.2%).[44] The degree to which religious and spiritual concerns are addressed by the care facility may substantially affect this relationship. Patients who reported that their spiritual needs were substantially supported by the medical team (26.3%) were significantly more likely to receive hospice care and less likely to receive aggressive care, and reported higher QOL. Pastoral care, received by about 46%, did not affect receiving such services, but did affect QOL just before death.[45] Patient-Oncologist EOL Discussions Recall of EOL discussions Recall of EOL discussions influences the EOL health care decisions and outcomes of patients with advanced cancer. One hundred twenty-three of 332 patients (37%) enrolled in CwC answered affirmatively when asked, "Have you and your doctors discussed any particular wishes you have about the care you would want to receive if you were dying?"[4] Recall of EOL discussions was associated with lower rates of mechanical ventilation, resuscitation, or ICU admission and earlier referral to hospice. A subsequent analysis reported on the treatment preferences of 325 patients who died while enrolled in CwC.[23] Seventy-two percent of patients with advanced cancer preferred treatment focused on comfort, answering affirmatively to the question, "If you could choose, would you prefer a plan of care that focused on relieving pain and discomfort, even if this meant not living as long?"; and 28% preferred life-extending treatment. Sixty-eight percent received EOL care consistent with baseline preferences. The likelihood of receiving care consistent with preferences was increased if the patient reported an EOL discussion with a physician (OR = 2.26; P < .0001) or were aware they were terminally ill (OR = 3.94; P = .0005). Recall of hospice discussions Hospice discussions increase the rate of hospice enrollment. As part of CanCORS, investigators interviewed 1,572 patients with stage IV lung cancer (or a surrogate if the patient was unable to participate for health reasons or had died) about whether they had discussed hospice with a health care provider before the interview.[48] Of the 1,517 patients in the final study cohort, 807 (53%) had discussed hospice with a physician before the interview. Hospice discussions strongly predicted enrollment in hospice within a year of diagnosis (70% enrollment with discussion vs. 26% without discussion; P < .001). The Nature of the Decision Decision to receive chemotherapy Patients with advanced cancer frequently receive multiple regimens of chemotherapy over the course of their treatment. Whether the decision involves first-line or second-line treatment for advanced disease may influence the decision-making process. One group of investigators conducted 117 semistructured interviews with 102 women with advanced breast cancer who were receiving first-line (n = 70) or second-line (n = 47) palliative chemotherapy.[31] Women in the second-line cohort were more likely than women in the first-line cohort to explain their decision to undergo chemotherapy because of the hope that it offered (43% vs. 19%; P = .006) and to report taking an active role in the decision process. Another group demonstrated that physicians exerted greater control over decisions when evidence for or against a treatment was less certain or if the cancer was advanced.[49] Thus, the patient's rationale for treatment and the relevance of the oncologist's perspective change with the nature of the decision. Decision to limit treatment Most deaths resulting from advanced cancer are preceded by decisions to limit treatment. Given the prevalence, importance, and challenges of these decisions, however, there is relatively little information about how patient preferences are taken into account during decision making. Using researchers embedded in health care teams, one group of investigators characterized the deliberations about limiting potentially life-prolonging treatments for 76 hospitalized patients with incurable cancer.[50] Two-thirds of the patients preferred comfort care, and one-third desired life-prolonging therapy. Patient preferences for comfort were more often in line with oncologists' preferences than were patient preferences for life-prolongation (91.4% vs. 46.7%; P = .001). Patients were involved in decisions to limit treatment only half of the time. Age, performance status, or decisional capacity did not influence the rate of patient involvement; agreement with oncologist preferences was the main predictor. Thus, decisions to limit treatment may reflect a shared perception rather than a shared decision-making process. References:
-
A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 274 (20): 1591-8, 1995 Nov 22-29.
-
Haidet P, Hamel MB, Davis RB, et al.: Outcomes, preferences for resuscitation, and physician-patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Am J Med 105 (3): 222-9, 1998.
-
Weeks JC, Cook EF, O'Day SJ, et al.: Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA 279 (21): 1709-14, 1998.
-
Wright AA, Zhang B, Ray A, et al.: Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300 (14): 1665-73, 2008.
-
Chen AB, Cronin A, Weeks JC, et al.: Palliative radiation therapy practice in patients with metastatic non-small-cell lung cancer: a Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J Clin Oncol 31 (5): 558-64, 2013.
-
Ayanian JZ, Chrischilles EA, Fletcher RH, et al.: Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol 22 (15): 2992-6, 2004.
-
American Cancer Society: Cancer Facts and Figures 2017. Atlanta, Ga: American Cancer Society, 2017. Available online. Last accessed May 25, 2017.
-
Elkin EB, Kim SH, Casper ES, et al.: Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol 25 (33): 5275-80, 2007.
-
Gagnon B, Mayo NE, Hanley J, et al.: Pattern of care at the end of life: does age make a difference in what happens to women with breast cancer? J Clin Oncol 22 (17): 3458-65, 2004.
-
Parr JD, Zhang B, Nilsson ME, et al.: The influence of age on the likelihood of receiving end-of-life care consistent with patient treatment preferences. J Palliat Med 13 (6): 719-26, 2010.
-
Fletcher K, Prigerson HG, Paulk E, et al.: Gender differences in the evolution of illness understanding among patients with advanced cancer. J Support Oncol 11 (3): 126-32, 2013.
-
Loggers ET, Maciejewski PK, Paulk E, et al.: Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol 27 (33): 5559-64, 2009.
-
Mack JW, Paulk ME, Viswanath K, et al.: Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med 170 (17): 1533-40, 2010.
-
Smith AK, Davis RB, Krakauer EL: Differences in the quality of the patient-physician relationship among terminally ill African-American and white patients: impact on advance care planning and treatment preferences. J Gen Intern Med 22 (11): 1579-82, 2007.
-
Smith AK, Earle CC, McCarthy EP: Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc 57 (1): 153-8, 2009.
-
Haas JS, Earle CC, Orav JE, et al.: Lower use of hospice by cancer patients who live in minority versus white areas. J Gen Intern Med 22 (3): 396-9, 2007.
-
Mack JW, Chen K, Boscoe FP, et al.: Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol 31 (20): 2569-79, 2013.
-
Sharma RK, Dy SM: Documentation of information and care planning for patients with advanced cancer: associations with patient characteristics and utilization of hospital care. Am J Hosp Palliat Care 28 (8): 543-9, 2011.
-
McCarthy EP, Burns RB, Ngo-Metzger Q, et al.: Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA 289 (17): 2238-45, 2003.
-
Craft PS, Burns CM, Smith WT, et al.: Knowledge of treatment intent among patients with advanced cancer: a longitudinal study. Eur J Cancer Care (Engl) 14 (5): 417-25, 2005.
-
Eidinger RN, Schapira DV: Cancer patients' insight into their treatment, prognosis, and unconventional therapies. Cancer 53 (12): 2736-40, 1984.
-
Yun YH, Kwon YC, Lee MK, et al.: Experiences and attitudes of patients with terminal cancer and their family caregivers toward the disclosure of terminal illness. J Clin Oncol 28 (11): 1950-7, 2010.
-
Mack JW, Weeks JC, Wright AA, et al.: End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 28 (7): 1203-8, 2010.
-
Pardon K, Deschepper R, Vander Stichele R, et al.: Are patients' preferences for information and participation in medical decision-making being met? Interview study with lung cancer patients. Palliat Med 25 (1): 62-70, 2011.
-
Gattellari M, Ward JE: Measuring men's preferences for involvement in medical care: getting the question right. J Eval Clin Pract 11 (3): 237-46, 2005.
-
Hawkins NA, Ditto PH, Danks JH, et al.: Micromanaging death: process preferences, values, and goals in end-of-life medical decision making. Gerontologist 45 (1): 107-17, 2005.
-
Fried TR, Bradley EH, O'Leary J: Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc 51 (10): 1398-403, 2003.
-
Hagerty RG, Butow PN, Ellis PM, et al.: Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis. J Clin Oncol 23 (6): 1278-88, 2005.
-
Hagerty RG, Butow PN, Ellis PM, et al.: Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol 16 (7): 1005-53, 2005.
-
Degner LF, Sloan JA: Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol 45 (9): 941-50, 1992.
-
Grunfeld EA, Maher EJ, Browne S, et al.: Advanced breast cancer patients' perceptions of decision making for palliative chemotherapy. J Clin Oncol 24 (7): 1090-8, 2006.
-
Bruera E, Sweeney C, Calder K, et al.: Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol 19 (11): 2883-5, 2001.
-
Kiely BE, Stockler MR, Tattersall MH: Thinking and talking about life expectancy in incurable cancer. Semin Oncol 38 (3): 380-5, 2011.
-
Koedoot CG, de Haan RJ, Stiggelbout AM, et al.: Palliative chemotherapy or best supportive care? A prospective study explaining patients' treatment preference and choice. Br J Cancer 89 (12): 2219-26, 2003.
-
Harrington SE, Smith TJ: The role of chemotherapy at the end of life: "when is enough, enough?". JAMA 299 (22): 2667-78, 2008.
-
Epstein RM, Peters E: Beyond information: exploring patients' preferences. JAMA 302 (2): 195-7, 2009.
-
Blinman P, King M, Norman R, et al.: Preferences for cancer treatments: an overview of methods and applications in oncology. Ann Oncol 23 (5): 1104-10, 2012.
-
Koedoot CG, Oort FJ, de Haan RJ, et al.: The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are. palliative chemotherapy and watchful-waiting. Eur J Cancer 40 (2): 225-35, 2004.
-
Stiggelbout AM, de Haes JC, Kiebert GM, et al.: Tradeoffs between quality and quantity of life: development of the QQ Questionnaire for Cancer Patient Attitudes. Med Decis Making 16 (2): 184-92, 1996 Apr-Jun.
-
Voogt E, van der Heide A, Rietjens JA, et al.: Attitudes of patients with incurable cancer toward medical treatment in the last phase of life. J Clin Oncol 23 (9): 2012-9, 2005.
-
Meropol NJ, Egleston BL, Buzaglo JS, et al.: Cancer patient preferences for quality and length of life. Cancer 113 (12): 3459-66, 2008.
-
Kaldjian LC, Curtis AE, Shinkunas LA, et al.: Goals of care toward the end of life: a structured literature review. Am J Hosp Palliat Care 25 (6): 501-11, 2008 Dec-2009 Jan.
-
Norton SA, Metzger M, DeLuca J, et al.: Palliative care communication: linking patients' prognoses, values, and goals of care. Res Nurs Health 36 (6): 582-90, 2013.
-
Phelps AC, Maciejewski PK, Nilsson M, et al.: Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA 301 (11): 1140-7, 2009.
-
Balboni TA, Paulk ME, Balboni MJ, et al.: Provision of spiritual care to patients with advanced cancer: associations with medical care and quality of life near death. J Clin Oncol 28 (3): 445-52, 2010.
-
Maciejewski PK, Phelps AC, Kacel EL, et al.: Religious coping and behavioral disengagement: opposing influences on advance care planning and receipt of intensive care near death. Psychooncology 21 (7): 714-23, 2012.
-
Pargament KI, Smith BW, Koenig HG, et al.: Patterns of positive and negative religious coping with major life stressors. J Sci Study Relig 37 (4): 710-24, 1998.
-
Huskamp HA, Keating NL, Malin JL, et al.: Discussions with physicians about hospice among patients with metastatic lung cancer. Arch Intern Med 169 (10): 954-62, 2009.
-
Keating NL, Beth Landrum M, Arora NK, et al.: Cancer patients' roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol 28 (28): 4364-70, 2010.
-
Winkler EC, Reiter-Theil S, Lange-Riess D, et al.: Patient involvement in decisions to limit treatment: the crucial role of agreement between physician and patient. J Clin Oncol 27 (13): 2225-30, 2009.
Potential Barriers to Planning the Transition to End-of-Life CareThe preferences of patients with advanced cancer should, in large part, determine the care they receive. However, the evidence reviewed in the section on Factors That Influence End-of-Life Care Decisions and Outcomes suggests that patients lack sufficient opportunity to develop informed preferences and, as a consequence, may seek care that is potentially inconsistent with their personal values and goals. This section identifies potential barriers that may prevent a patient with advanced cancer and his or her oncologist from discussing prognosis, goals, options, and preferences.[1] The information will allow the oncology clinician to develop the strategies needed to approach these challenging discussions more deliberately. Potential barriers include the following: - Patients' interpretations of prognostic information.
- Lack of agreement between patients and oncologists.
- Oncologists' communication behaviors.
- Oncologists' misconceptions about the harm of end-of-life (EOL) discussions.
- Oncologists' attitudes and preferences.
- Reimbursement for chemotherapy and practice economics.
- Uncertainty about options other than disease-directed treatments.
Patients' Interpretations of Prognostic Information A consistent finding over the last two decades is that patients with advanced cancer are typically overly optimistic about their life expectancies or the potential for cure with cancer-directed therapies. - One group of investigators studied 1,193 patients in the Cancer Care Outcomes Research and Surveillance Consortium and found that a significant majority of patients with advanced lung or colorectal cancer did not understand that treatment was not curative. Sixty-nine percent of patients did not report understanding that chemotherapy was not at all likely to cure their cancer.[2] Patients who were nonwhite, were diagnosed with colorectal cancer, or reported satisfaction with physician communication were more likely to report inaccurate understanding of treatment intent.
- Similarly, 64% of patients with incurable lung cancer who received radiation did not understand that radiation was not at all likely to cure them. Older patients and nonwhites were more likely to misunderstand; surrogates of patients were more likely to understand.[3]
There are many potential barriers to a more accurate understanding of prognosis, including poor communication by oncology clinicians. However, patients also interpret information for reasons unrelated to the quality of communication. The perspectives of patients with advanced cancer who enroll in phase I clinical trials or surrogate decision makers for patients in intensive care units (ICUs) provide some insights into why advanced cancer patients might misinterpret prognostic information. - Patients' optimistic expectations of benefit from phase I trials were associated with a better quality of life, stronger religious faith, optimism, poorer numeracy (ability to understand a statistical estimate of treatment outcome), and monetary risk seeking. They were unrelated to age, gender, educational level, or functional status.[4]
- In a study of 163 patients enrolled in a phase I trial, most were aware of hospice (81%) or palliative care (84%), but few considered either choice seriously (hospice, 10%; palliative care, 7%). Seventy-five percent of patients reported the most important influence was awareness that their cancer was growing; 63% percent stated the knowledge that the phase I drug killed cancer cells was the most important factor in their decision to enroll.[5]
- In a study of 80 surrogate decision makers recruited from the families of ICU patients, most were fairly accurate in their interpretations of quantitative information and less ambiguous qualitative estimates provided by ICU physicians. However, several potentially relevant sources of prognostic misunderstanding included the need to express optimism, the belief that the patients' fortitude would lead to better-than-predicted outcomes, and a disbelief that physicians can predict accurately.[6]
Lack of Agreement Between Patients and Oncologists Multiple conversations between patients with advanced cancer and their oncologists should lead to an understanding about prognoses, goals, preferences, options, and the decision-making process. However, evidence suggests that patients and oncologists frequently do not reach the same conclusions about these issues. How this lack of agreement affects the transition to EOL care is not certain. However, it is unlikely that disagreement with or misperceptions about goals of treatment, for example, can contribute positively to the timely planning for the transition to EOL care. Understanding of prognosis One group of investigators analyzed the prognostic estimates of 917 adults with metastatic colorectal or lung cancer who were enrolled in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) and their physicians.[7] There were three notable findings: - Patients were more optimistic than physicians.
- Physician estimates were more calibrated with the observed survival than were the estimates of patients.
- Patients who were more optimistic than their oncologists were more likely to prefer life-extending treatments.
The poor concordance between patients and oncology clinicians has been observed in a diverse range of patients, including patients with acute myeloid leukemia [8] or those who are considering allogeneic stem cell transplantation.[9] (Refer to the Patients' Interpretations of Prognostic Information section of this summary for additional information about patient prognostic understanding.) Goals of treatment Patients and oncologists frequently do not share an understanding of the goals of cancer treatment. One group of investigators reported that in 25% of the advanced-cancer patient-physician dyads surveyed, the patient thought the goal of care was to cure disease when it was not.[10] Younger patients, patients who were native English speakers, and patients who received written information were more likely to agree with their oncologists about the intent of chemotherapy. Another group of researchers reported that 64% of patients with advanced-stage lung cancer did not understand that the radiation therapy prescribed was not curative.[3] Topics recalled from consultation Researchers reported that patients and oncologists frequently recalled different components of communication and decision making from discussions of phase I trials.[11] For example, 13 of 17 participating oncologists mentioned prognosis in fewer than 50% of consultations. Although oncologists frequently reported discussing prognosis, only 12% of patients and 20% of independent researchers who coded the recorded consultations agreed. There was better agreement about unknown adverse events and the voluntary nature of trial enrollment. Patient preference for decision-making role Oncology clinicians frequently do not correctly identify patient preferences for decision-making roles. Results from a few Illustrative studies are summarized below. - In a study of older patients with metastatic colorectal cancer, 52% preferred a passive role. However, oncologists correctly identified patient preferences only 41% of the time.[12]
- Palliative care physicians correctly identified the decision-making preference of patients with advanced cancer in a palliative care clinic only 38% of the time.[13]
Assessment of patient performance status Physicians evaluate patient performance status (PS) in determining prognosis and in making treatment decisions. Investigators compared the Eastern Cooperative Oncology Group PS reported by physicians and 1,636 patients who had advanced lung or colorectal cancers.[14] They demonstrated 56.6% disagreement between patients and physicians about PS; more optimism by physicians about PS (mean, 0.91 vs. 1.30); and association of disagreement with an increased risk of death. Patient preference for cardiopulmonary resuscitation The initial phase of SUPPORT demonstrated that only 47% of physicians knew when their patients wanted to avoid cardiopulmonary resuscitation (CPR).[15] In a subsequent analysis, researchers studied 520 patients with metastatic colorectal cancer.[16] Information about CPR preferences for 339 of the patients was available: 223 (63%) wanted CPR; however, physicians incorrectly identified patient preference in 30% of cases. The preference for CPR was stronger among patients who had a more optimistic sense of the likelihood of survival at 2 months. Other investigators found similar results in a group of patients with lung disease.[17] Physicians frequently misunderstood patients' preferences (25%-40% of dyads). The lack of agreement was greater if patients wished to avoid resuscitation. Neither study reported the effect of the disagreement on patient outcomes. Oncologist Communication Behaviors This section summarizes the evidence that identifies deficits in the communication behaviors of oncologists treating patients who have advanced cancer. The evidence cited here strongly suggests that doctor-patient communication frequently does not fully support informed or shared decision making. This information will allow oncology clinicians to reflect on their communication habits and consider modifying impediments to the timely planning for the transition to EOL care. Observational studies of patient-oncologist communication Investigators reported a study of audiotaped consultations between one of nine oncologists and 118 patients with advanced cancer.[18] They devised a coding system to assess how frequently essential information (e.g., aim of treatment, incurability of cancer, risks of treatment, information about life expectancy, and alternative treatment options) was disclosed and how much the doctor facilitated the patient's participation in making decisions. A summary of results follows, with independent observations cited as appropriate. - Most oncologists disclosed the noncurative intent of chemotherapy in advanced cancer, but few discussed alternatives to chemotherapy. In 74.6% of encounters, patients were told the cancer was incurable. However, patients were presented with information about life expectancy in only 57.6% of encounters, and were given information about treatment alternatives in only 44.6% of encounters.
- Oncologists rarely checked patient understanding. Oncologists asked about patient understanding of the disclosed information and decision-making process in only 10% of encounters.
- Essential elements of shared decision making were frequently missing. The participating oncologists frequently acknowledged the uncertainty of treatment benefit (72.9%), discussed trade-offs in receiving treatment (60.2%), and elicited patient perspectives about treatment (69.5%). However, only 29.7% of patients were offered a choice, and in only 10.2% of the encounters did the oncologist assess the patient's comprehension.
Additional deficits in oncologist communication behaviors include the following: - Oncologists frequently use ambiguous or falsely reassuring language, such as inappropriately optimistic statements. One group of researchers analyzed audiotapes of encounters between oncologists and patients in which the oncologists had provided a likelihood of cure using current treatments.[19] The audiotapes were selected according to the degree of prognostic agreement and then coded to determine factors that predict agreement. Oncologists were more likely to make optimistic statements, but pessimistic statements were more likely to increase the degree of prognostic agreement. The authors concluded that the best communication strategy may include acknowledging that the tendency to be optimistic may interfere with patient understanding of prognosis, and striving to provide honest information as warranted by the prognosis.
- Oncologists frequently do not discuss the anticipated survival benefit of chemotherapy in advanced cancer. One group of investigators demonstrated that in 26 of 37 analyzed consultations, the oncologist did not provide the patient with information about the potential survival gain of palliative (i.e., noncurative) chemotherapy.[20]
Oncologist self-reported practices in prognostic communication There is evidence that physicians' attitudes toward prognostic communication influence patients' prognostic awareness. In an analysis of physician surveys from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium,[21] investigators reported that patients with metastatic lung or colorectal cancer were more likely to have an accurate prognostic awareness if their most-important doctor reported discussing prognosis earlier rather than waiting for deterioration (18.5% vs. 7.6%; odds ratio, 3.23; 95% confidence interval, 1.39-7.52; P = .006). Thus, understanding the factors that influence oncologists' attitudes is relevant to improving prognostic communication. Two additional surveys of American oncologists and communication about prognosis have been published. One study analyzed the survey responses of 729 oncologists (64% response rate).[22] Almost all (98%) indicated they would disclose a terminal prognosis, but 48% indicated they would do so only when the patient's preference for disclosure of prognosis was known. Fewer than half (43%) always provided an estimate of time until death. Three-quarters indicated they had not received formal training in communication of terminal prognoses; 96% thought training should be mandatory. Another study reported that 65% of physicians surveyed discussed prognosis immediately with asymptomatic patients who had advanced cancer and anticipated life expectancies of 4 to 6 months.[23] However, fewer physicians would immediately discuss resuscitation preference (44%), hospice (26%), or preferred site of death (21%), with most physicians waiting for patient symptoms to appear or until there were no more treatments to offer. Younger physicians, surgeons, and oncologists were more likely than noncancer specialists to discuss prognosis. Oncologists' Misconceptions About the Harm of EOL Discussions Oncologists cite several reasons for their reluctance to engage in EOL discussions. However, several studies have provided evidence that many of the concerns-e.g., causing psychological harm or destroying hope-are not valid. On the contrary, EOL conversations are not psychologically harmful and may improve psychological adjustment, while explicit information about prognosis does not necessarily compromise a patient's sense of hope. - A multisite, prospective, longitudinal cohort study reported that discussions about EOL care were not psychologically harmful. Patients with advanced cancer who answered affirmatively to the question, "Have you and your doctors discussed any particular wishes you have about the care you would want to receive if you were dying?" did not have a higher rate of generalized anxiety disorder or major depressive disorder than did patients who did not recall such discussions (3.3% vs. 1.4% and 8.3% vs. 5.8%, respectively).[24] In addition, recall of such discussions was not related to levels of worry.
- Another study found that patient anxiety caused by disclosure of information and decision making was transient or may have improved psychological adjustment. The amount of information disclosed by oncologists did not predict levels of patient anxiety.[18] However, greater encouragement of the patient to participate in decision making was independently associated with higher anxiety levels immediately after the consultation and 2 weeks later.
In another study, men with advanced cancer who estimated a lower likelihood of survival at 6 months had increased levels of anxiety and depression.[25] However, men who reported having a full discussion about prognosis with their oncologist had less depression and similar levels of anxiety. Thus, discussions about prognosis moderated the relationships with anxiety and depression and may have facilitated long-term psychological adjustment. - A study found that explicit disclosure about prognosis and reassurance about nonabandonment were helpful to patients. Researchers studied how patients with breast cancer and healthy women reacted to two levels of prognostic disclosure and reassurance portrayed in four different video vignettes.[26] Explicitness and reassurance decreased uncertainty and anxiety. The authors cautioned that the nature of the findings is experimental.
Oncologist Attitudes and Preferences This section summarizes the information strongly suggesting that oncologists' attitudes and preferences influence their communication and decision-making behaviors in a manner that might change patients' EOL decisions. One study found that hospital staff attributed variations in aggressiveness of care near the EOL to physician characteristics, including physician beliefs, attitudes, and socialization within the practice of medicine.[27] As a result, communication about advance directives, prognosis, limiting treatment near the EOL, and acknowledgment that the patient was dying varied significantly among physicians. A 2011 study demonstrated that physicians may recommend different treatments for patients than they would choose for themselves.[28] This section encourages oncology clinicians to reflect on these biases and how to minimize their effects on communication behaviors. Oncologist preferences for noncurative treatments Oncology clinicians influence patients' understanding of treatment preferences. Dutch researchers surveyed medical oncologists about their preferences for palliative (noncurative) chemotherapy or observation using case vignettes.[29] The gender, age, and employment status of the oncologist and type of hospital did not influence preferences for chemotherapy. However, older patients or patients without a stated preference were less likely to receive chemotherapy. There was a greater preference for chemotherapy if the anticipated survival gain was at least 3 months, if the treatment was mildly toxic, or if symptoms of disease progression were identified. These findings are consistent with a study of U.S. oncologists that demonstrated a preference for life-sustaining treatment rather than comfort care.[30] Oncologists and shared decision making In surveys, oncologists are broadly supportive of the concept of shared decision making. However, empirical research demonstrates that oncologists' communication behaviors frequently do not support shared decision making. One group of investigators interviewed Australian cancer specialists about their inclusion of patients in decision making and identified several factors that influence patient involvement.[31] Disease-related factors included stage of disease, availability of treatment options, and risks to the patient. In addition, the public perception of the disease and whether there was a clear best option were relevant. Cancer specialists were more likely to include patients in decision making when the disease stage was advanced and treatment options were less certain. Patient characteristics that decreased doctors' efforts to involve the patient included: - Increased anxiety.
- Older age.
- Female gender.
- A Mediterranean or Central or Eastern European background.
- A busy professional life.
The doctors were aware of patient preferences for involvement but felt most patients deferred to their expertise. Furthermore, few physicians had a validated approach to determine patient preferences. Oncologist attitudes toward EOL care Attitudes toward EOL care may also influence the communication and decision-making behaviors of oncologists. In a qualitative study of 18 academic oncologists who were asked to reflect on recent patient deaths, one group of researchers reported that oncologists who viewed EOL care as an important part of their job reported increased job satisfaction.[32] Other investigators reported on a survey of Japanese oncologists to assess the level of burden experienced by oncologists when recommending discontinuation of anticancer treatment.[33] Forty-seven percent reported high levels of burden. Multivariate analysis of determinants of the sense of burden identified the following covariates: - Feeling that the patient was being deprived of hope.
- Concerns that the family would blame the doctor.
- Concerns that the patient would lose control.
- Concerns that there was inadequate time to discuss the recommendation.
Reimbursement for Chemotherapy and Practice Economics Before 2003, reimbursement for chemotherapy was at a price that greatly exceeded acquisition costs for medical oncologists. Although the profit margin for chemotherapy has decreased, chemotherapy remains a significant source of oncologists' income. Researchers demonstrated that physicians' decisions to prescribe chemotherapy for patients with advanced cancer was not affected by reimbursement rates, but more costly regimens were more likely with higher rates of reimbursement.[34] Additional results are summarized below: - Urologists who acquired ownership in intensity-modulated radiation therapy (IMRT) increased their utilization of IMRT (from 13.1% to 32.3%), compared with urologists who did not own IMRT services (from 14.3% to 15.6%).[35] The rate at National Comprehensive Cancer Network cancer centers was unchanged at 8%.
- Oncologists who practice in a fee-for-service setting or those who are paid a salary with productivity incentives are more likely to report that their income increases with increases in ordering chemotherapy or growth factors.[36]
Uncertainty About Options Other Than Disease-Directed Treatments A final barrier to planning the transition to EOL care may be confusing language when patients begin to ponder forgoing resuscitation and other life-prolonging interventions. On the basis of their experiences or understanding, oncology clinicians, patients, and families assign different-and often valid-meanings to terms such as supportive or palliative. A 2013 systematic review of the literature found widespread inconsistencies in the definitions of supportive care, palliative care, and hospice.[37] The section on Supportive Care, Palliative Care, and Hospice in Advanced Cancer defines terms more completely and discusses clearly communicating the purpose of each level of care relevant to the patient with advanced cancer. References:
-
Quill TE, Holloway RG: Evidence, preferences, recommendations--finding the right balance in patient care. N Engl J Med 366 (18): 1653-5, 2012.
-
Weeks JC, Catalano PJ, Cronin A, et al.: Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med 367 (17): 1616-25, 2012.
-
Chen AB, Cronin A, Weeks JC, et al.: Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol 31 (21): 2730-5, 2013.
-
Weinfurt KP, Castel LD, Li Y, et al.: The correlation between patient characteristics and expectations of benefit from Phase I clinical trials. Cancer 98 (1): 166-75, 2003.
-
Agrawal M, Grady C, Fairclough DL, et al.: Patients' decision-making process regarding participation in phase I oncology research. J Clin Oncol 24 (27): 4479-84, 2006.
-
Zier LS, Sottile PD, Hong SY, et al.: Surrogate decision makers' interpretation of prognostic information: a mixed-methods study. Ann Intern Med 156 (5): 360-6, 2012.
-
Weeks JC, Cook EF, O'Day SJ, et al.: Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA 279 (21): 1709-14, 1998.
-
Sekeres MA, Stone RM, Zahrieh D, et al.: Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia 18 (4): 809-16, 2004.
-
Lee SJ, Fairclough D, Antin JH, et al.: Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA 285 (8): 1034-8, 2001.
-
Lennes IT, Temel JS, Hoedt C, et al.: Predictors of newly diagnosed cancer patients' understanding of the goals of their care at initiation of chemotherapy. Cancer 119 (3): 691-9, 2013.
-
Jenkins V, Solis-Trapala I, Langridge C, et al.: What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol 29 (1): 61-8, 2011.
-
Elkin EB, Kim SH, Casper ES, et al.: Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol 25 (33): 5275-80, 2007.
-
Bruera E, Sweeney C, Calder K, et al.: Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol 19 (11): 2883-5, 2001.
-
Schnadig ID, Fromme EK, Loprinzi CL, et al.: Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer 113 (8): 2205-14, 2008.
-
A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 274 (20): 1591-8, 1995 Nov 22-29.
-
Haidet P, Hamel MB, Davis RB, et al.: Outcomes, preferences for resuscitation, and physician-patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Am J Med 105 (3): 222-9, 1998.
-
Downey L, Au DH, Curtis JR, et al.: Life-sustaining treatment preferences: matches and mismatches between patients' preferences and clinicians' perceptions. J Pain Symptom Manage 46 (1): 9-19, 2013.
-
Gattellari M, Voigt KJ, Butow PN, et al.: When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol 20 (2): 503-13, 2002.
-
Robinson TM, Alexander SC, Hays M, et al.: Patient-oncologist communication in advanced cancer: predictors of patient perception of prognosis. Support Care Cancer 16 (9): 1049-57, 2008.
-
Audrey S, Abel J, Blazeby JM, et al.: What oncologists tell patients about survival benefits of palliative chemotherapy and implications for informed consent: qualitative study. BMJ 337: a752, 2008.
-
Liu PH, Landrum MB, Weeks JC, et al.: Physicians' propensity to discuss prognosis is associated with patients' awareness of prognosis for metastatic cancers. J Palliat Med 17 (6): 673-82, 2014.
-
Daugherty CK, Hlubocky FJ: What are terminally ill cancer patients told about their expected deaths? A study of cancer physicians' self-reports of prognosis disclosure. J Clin Oncol 26 (36): 5988-93, 2008.
-
Keating NL, Landrum MB, Rogers SO Jr, et al.: Physician factors associated with discussions about end-of-life care. Cancer 116 (4): 998-1006, 2010.
-
Wright AA, Zhang B, Ray A, et al.: Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300 (14): 1665-73, 2008.
-
Cripe LD, Rawl SM, Schmidt KK, et al.: Discussions of life expectancy moderate relationships between prognosis and anxiety or depression in men with advanced cancer. J Palliat Med 15 (1): 99-105, 2012.
-
van Vliet LM, van der Wall E, Plum NM, et al.: Explicit prognostic information and reassurance about nonabandonment when entering palliative breast cancer care: findings from a scripted video-vignette study. J Clin Oncol 31 (26): 3242-9, 2013.
-
Larochelle MR, Rodriguez KL, Arnold RM, et al.: Hospital staff attributions of the causes of physician variation in end-of-life treatment intensity. Palliat Med 23 (5): 460-70, 2009.
-
Ubel PA, Angott AM, Zikmund-Fisher BJ: Physicians recommend different treatments for patients than they would choose for themselves. Arch Intern Med 171 (7): 630-4, 2011.
-
Koedoot CG, De Haes JC, Heisterkamp SH, et al.: Palliative chemotherapy or watchful waiting? A vignettes study among oncologists. J Clin Oncol 20 (17): 3658-64, 2002.
-
Kozminski MA, Neumann PJ, Nadler ES, et al.: How long and how well: oncologists' attitudes toward the relative value of life-prolonging v. quality of life-enhancing treatments. Med Decis Making 31 (3): 380-5, 2011 May-Jun.
-
Shepherd HL, Butow PN, Tattersall MH: Factors which motivate cancer doctors to involve their patients in reaching treatment decisions. Patient Educ Couns 84 (2): 229-35, 2011.
-
Jackson VA, Mack J, Matsuyama R, et al.: A qualitative study of oncologists' approaches to end-of-life care. J Palliat Med 11 (6): 893-906, 2008.
-
Otani H, Morita T, Esaki T, et al.: Burden on oncologists when communicating the discontinuation of anticancer treatment. Jpn J Clin Oncol 41 (8): 999-1006, 2011.
-
Jacobson M, O'Malley AJ, Earle CC, et al.: Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 25 (2): 437-43, 2006 Mar-Apr.
-
Mitchell JM: Urologists' use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med 369 (17): 1629-37, 2013.
-
Malin JL, Weeks JC, Potosky AL, et al.: Medical oncologists' perceptions of financial incentives in cancer care. J Clin Oncol 31 (5): 530-5, 2013.
-
Hui D, De La Cruz M, Mori M, et al.: Concepts and definitions for "supportive care," "best supportive care," "palliative care," and "hospice care" in the published literature, dictionaries, and textbooks. Support Care Cancer 21 (3): 659-85, 2013.
Supportive Care, Palliative Care, and Hospice in Advanced CancerImportance of a Name Oncologists' attitudes toward palliative care The European Society of Medical Oncology surveyed its members about their attitudes toward and involvement in palliative care for patients with advanced cancer.[1] Eighty-eight percent of respondents endorsed the belief that medical oncologists should coordinate end-of-life (EOL) care, but 42% felt they were inadequately trained for the task. Relatively few respondents collaborated with a palliative care specialist (35%) or inpatient hospice services (26%). The term palliative care has been identified through surveys as a potential impediment to referral to a palliative care clinic.[2] A 2013 report from a single institution that changed the name of its palliative care service to supportive care service demonstrated that outpatients were referred sooner after first hospital registration (median, 9.2 months vs. 13.2 months; P < .001) and sooner after the first diagnosis of advanced cancer (median, 5.2 months vs. 6.9 months; P < .001).[3] Public attitudes toward palliative care Public attitudes toward palliative care depend on the description of the service. A 2011 public opinion poll conducted with more than 100 consumers found a lack of knowledge about palliative care among more than 75% of interviewees.[4] After the opinion poll was taken, the definition of palliative care was revised for the public. In the new definition, palliative care is described as "specialized medical care for people with serious illnesses." The focus of care is defined as relief from pain and other symptoms, regardless of diagnosis. Included in the definition are patient and family quality of life (QOL), the interdisciplinary team of specialists, and the statement that palliative care is appropriate at any age, at any stage of illness, and in conjunction with curative treatment.[4] Best Supportive Care The ability to safely administer multiple cycles of chemotherapy depends on a range of interventions to minimize adverse effects. Most oncologists recognize that anticipating, recognizing, and responding to common adverse events with antiemetics, hematopoietic growth factors to reduce the occurrence of red cell transfusions or risks of neutropenia-associated infections, transfusions, medications to alleviate anxiety or depression, and analgesics for pain are mandatory skill sets in medical oncology. In this context, supportive care is an adjunct to the goal of maximizing the benefits of disease-directed treatment while minimizing potential decline in a patient's health-related QOL. Three points about the term supportive care are relevant to this section: - Inadequate attention is paid to symptoms or health-related QOL during routine oncology evaluations. Although oncologists may overestimate their skillfulness, it is prudent to refer patients with burdensome symptoms to physicians who have a specific interest in health-related QOL and who have expertise in symptom management. The goal is always optimal supportive care; at times, a palliative care specialist may be required.
- The term best supportive care has been used to describe the care received by patients when they were no longer receiving disease-directed treatment. It is unlikely that best supportive care-even when defined within protocols-would be confused with the specialized palliative care provided to patients with advanced cancer.
- Using the term supportive care may be preferable for referring clinicians and their patients because it avoids the negative connotation of the term palliative care.
Palliative Care Palliative care is an interdisciplinary model of care focused on patients with serious or life-threatening illnesses and their families. The goals of palliative care are to: - Reduce illness burden, relieve suffering, and maintain QOL through interventions that maintain physical, psychological, social, and spiritual well-being.
- Improve communication and care coordination.
- Ensure that care is consistent with the values and preferences of the patient.
- Ensure that dying occurs with minimal suffering and maximum opportunities for closure.
Palliative care is generally provided by physicians, nurses, social workers, and/or chaplains. Palliative care teams typically focus on alleviation of uncontrolled symptoms, goals of care, distress related to the process of dying, and family burden. Palliative care is both a philosophy of care and an organized, highly structured system for delivering care. Palliative care goes beyond the traditional disease model to include goals of care such as the following: - Enhanced QOL.
- Optimization of function.
- Informed and active decision making.
- Opportunities for closure and personal growth.
Palliative care can be delivered concurrently with life-prolonging care or as the main focus of care. (Refer to the Integrating Palliative Care Into Conventional Cancer Care section in the Strategies to Improve Patient-Oncologist Communication and Decision Making in Advanced Cancer section of this summary for more information.) The term palliative care has often been used to communicate the noncurative intent of chemotherapy for patients with advanced cancer or to signal, without clarifying the intent, the transition away from chemotherapy. This use may contribute to the persistent and pervasive sense that palliative care is equivalent to EOL care. However, palliative care is a specialized approach to the care of patients with serious or life-threatening illnesses; it is guided by principles and accomplished via a specific-but by no means exclusive-skill set distinct from the skills required to prescribe and manage chemotherapy. Hospice Hospice is a team-oriented approach to providing expert medical care, pain management, and emotional and spiritual support for patients whose life expectancy is no longer than 6 months. Most hospice care is delivered in the home. Hospice eligibility criteria The criteria for hospice eligibility are established by state and federal regulations. A licensed physician needs to certify that the patient is suffering from a life-limiting illness, with a life expectancy of no longer than 6 months. The patient must sign a statement choosing hospice instead of other forms of treatment. In addition, the patient must receive care from a Medicare-approved hospice program. Hospice services are covered by Medicare, Medicaid, managed care, private health insurance, other third-party payers, and private pay. Hospice may be indicated when a patient who has cancer: - Presents with distant metastatic disease.
- Has progressed from an earlier stage of illness to metastatic disease and:
- Shows continued decline in spite of therapy.
- Refuses active treatment.
- Desires palliative and hospice care.
Patients who have poor prognoses, such as those who have pancreatic and brain cancers, can be eligible for hospice without meeting these criteria.[5] Patient and caregiver barriers to hospice enrollment The following points summarize the many potential barriers to timely hospice enrollment: - Patients and caregivers assume that hospice is only for the final days of life.
- Preference for life-sustaining treatments may dissuade many patients from hospice enrollment.[6]
- Many patients believe that hospice means giving up hope.
- Denial of declining health status may pose a barrier to patients' willingness to consider hospice.
- Patients and families may have negative perceptions of hospice.[7]
- Patient demographics affect their perceptions of hospice.
- New anticancer therapies provide patients with new options and reasons for optimism, even though the actual clinical benefits may be few.[8]
Physician barriers to hospice enrollment The following points summarize physician barriers to hospice enrollment: - Oncologists have difficulty making accurate predictions about estimated life expectancy and do not offer patients the opportunity to learn about hospice.[9]
- Referral to hospice may be interpreted as professional failure by the oncologist.[10]
- Referral to hospice may signify unwelcome loss of control for the oncologist.[11]
- A trend toward late hospice referral leads to burdensome transitions and patients spending 3 days or fewer in hospice.[12]
- Oncologists may believe that hospice enrollment will hasten death; however, data do not support this concern.[13] One group of investigators reported on the survival of 4,493 patients from a sample of Medicare beneficiaries for 1998-2002.[14] Hospice enrollment was not significantly associated with shorter survival when compared with nonhospice patients' survival. Instead, the mean survival was significantly longer for hospice patients with lung and pancreatic cancer and marginally longer for hospice patients with colon cancer.
A 2011 observational study from South Korea demonstrated that patients who were aware of their terminal illness and admitted to an inpatient palliative care unit had a median survival of 69 days. Survival was unaffected by admission to the intensive care unit, suggesting that patient-level characteristics, rather than medical interventions, influence survival.[15] - Oncologists fear that a hospice referral will be seen by the patient as abandonment.
Potential shortcomings of the hospice system Studies suggest that the hospice requirement that patients forgo disease-directed, potentially life-sustaining treatments may deprive patients in need of services. In one study of 300 cancer patients and 171 family members, willingness to forgo treatment was not well correlated with greater need for hospice services.[16] Instead, independent predictors of patients' perceived need for hospice services were being African American and having lower social support, worse functional status, and higher psychological symptom burden. For families, predictors included caregiver burden, worse self-reported health, working outside the home, and caring for a cancer patient with worse functional status. The authors suggest that the criterion of ending life-sustaining treatment for hospice be reconsidered.[16] Hospices' own enrollment policies may contribute to underuse of the benefit in the United States. Results from a national survey of 591 U.S. hospices showed that 78% had at least one enrollment policy restricting access to care for patients with high-cost medical care needs, such as chemotherapy, radiation, transfusions, and total parenteral nutrition. Such policies are more likely to be found at smaller hospices, for-profit hospices, and hospices in rural areas of the country.[17] Whether to define such treatment as curative or palliative is often unclear. In addition, for-profit hospices are less likely to admit patients with shorter, and less profitable, life expectancies.[18] Hospice and resuscitation and other potentially life-sustaining interventions While a do-not-resuscitate status is not mandatory for enrollment in hospice, less aggressive medical care near the EOL is associated with higher patient-reported QOL and better bereavement adjustment.[19] Thus, it is important to clarify patient preferences at the time of hospice discussions and, to the extent possible, agree on a plan of care. References:
-
Cherny NI, Catane R; European Society of Medical Oncology Taskforce on Palliative and Supportive Care: Attitudes of medical oncologists toward palliative care for patients with advanced and incurable cancer: report on a survery by the European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Cancer 98 (11): 2502-10, 2003.
-
Fadul N, Elsayem A, Palmer JL, et al.: Supportive versus palliative care: what's in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 115 (9): 2013-21, 2009.
-
Dalal S, Palla S, Hui D, et al.: Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist 16 (1): 105-11, 2011.
-
Center to Advance Palliative Care: 2011 Public Opinion Research on Palliative Care: A Report Based on Research by Public Opinion Strategies. New York, NY: The Center to Advance Palliative Care, 2011. Available online. Last accessed February 27, 2017.
-
End-Stage Indicators: Disease-Specific Guidelines. Rockville, Md: Montgomery Hospice, 2013. Available online. Last accessed February 27, 2017.
-
Casarett D, Van Ness PH, O'Leary JR, et al.: Are patient preferences for life-sustaining treatment really a barrier to hospice enrollment for older adults with serious illness? J Am Geriatr Soc 54 (3): 472-8, 2006.
-
McCarthy EP, Burns RB, Davis RB, et al.: Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol 21 (4): 728-35, 2003.
-
Mintzer DM, Zagrabbe K: On how increasing numbers of newer cancer therapies further delay referral to hospice: the increasing palliative care imperative. Am J Hosp Palliat Care 24 (2): 126-30, 2007 Apr-May.
-
Thomas JM, O'Leary JR, Fried TR: Understanding their options: determinants of hospice discussion for older persons with advanced illness. J Gen Intern Med 24 (8): 923-8, 2009.
-
McGorty EK, Bornstein BH: Barriers to physicians' decisions to discuss hospice: insights gained from the United States hospice model. J Eval Clin Pract 9 (3): 363-72, 2003.
-
Brickner L, Scannell K, Marquet S, et al.: Barriers to hospice care and referrals: survey of physicians' knowledge, attitudes, and perceptions in a health maintenance organization. J Palliat Med 7 (3): 411-8, 2004.
-
Teno JM, Gozalo PL, Bynum JP, et al.: Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 309 (5): 470-7, 2013.
-
Saito AM, Landrum MB, Neville BA, et al.: Hospice care and survival among elderly patients with lung cancer. J Palliat Med 14 (8): 929-39, 2011.
-
Connor SR, Pyenson B, Fitch K, et al.: Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage 33 (3): 238-46, 2007.
-
Yun YH, Lee MK, Kim SY, et al.: Impact of awareness of terminal illness and use of palliative care or intensive care unit on the survival of terminally ill patients with cancer: prospective cohort study. J Clin Oncol 29 (18): 2474-80, 2011.
-
Casarett DJ, Fishman JM, Lu HL, et al.: The terrible choice: re-evaluating hospice eligibility criteria for cancer. J Clin Oncol 27 (6): 953-9, 2009.
-
Aldridge Carlson MD, Barry CL, Cherlin EJ, et al.: Hospices' enrollment policies may contribute to underuse of hospice care in the United States. Health Aff (Millwood) 31 (12): 2690-8, 2012.
-
Lindrooth RC, Weisbrod BA: Do religious nonprofit and for-profit organizations respond differently to financial incentives? The hospice industry. J Health Econ 26 (2): 342-57, 2007.
-
Wright AA, Zhang B, Ray A, et al.: Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300 (14): 1665-73, 2008.
Strategies to Improve Patient-Oncologist Communication and Decision Making in Advanced CancerSeveral strategies can potentially improve the quality of oncologist-patient communication and decision making and facilitate the transition to end-of-life (EOL) care for patients who have advanced cancer. (Refer to the Training in Communication Skills section in the PDQ summary on Communication in Cancer Care for more information about workshops designed to improve the communication skills of oncology clinicians.) This section summarizes information relevant to the following strategies: - Improving prognostication or prognostic communication.
- Promoting advance care planning.
- Providing patients with materials to prepare for consulting with the oncologist.
- Utilizing decision aids.
- Integrating specialized palliative care services into conventional cancer care.
Improving Prognostication A comprehensive review of various strategies to improve prognostication is beyond the scope of this summary. One study has recently published a concise overview.[1] There are several sources of prognostic information: - Clinical predictions of survival.
- Patient performance status.
- Patient-reported health-related quality of life (QOL), which is a prognostic factor in patients with advanced cancer. In one study of patients with locally advanced non-small cell lung cancer (NSCLC), scores on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) replaced the classic prognostic factors, including Karnofsky Performance Status (KPS), on multivariate analysis.[2] Patients with EORTC QLQ-C30 scores of 66.7 or higher had a median overall survival (OS) of 27.1 months, compared with 15.4 months for patients with lower scores. However, a single measure of QOL may be sufficient.
Other investigators reported on the prognostic impact of a single self-reported QOL item in a cohort of almost 2,500 patients with newly diagnosed lung cancer.[3] Patients with a clinically significant QOL deficit had a median survival of 1.6 years, compared with 5.6 years without a deficit. Older age, worse performance status, and advanced stage of disease were also significant predictors; the QOL measure maintained independent influence on multivariable analysis. - Formal prognostic systems using multiple variables.
Novel Strategies to Improve Communication of Prognosis An Australian study demonstrated that most patients with metastatic cancer wanted information about life expectancy or prognosis; some patients thought that receiving estimates of worst-, typical-, and best-case outcomes would be acceptable.[4] Other investigators proposed that percentiles derived from multiple published OS curves for metastatic cancer would serve as the basis for estimating best- and worst-case scenarios.[5] Their subsequent work has established the following: - The method is reliable and accurate. For front-line chemotherapy in metastatic breast cancer or NSCLC, researchers could accurately estimate outcomes by using simple multiples of the median derived from an OS curve.[6] For example, for patients with metastatic breast cancer, the worst-case scenario for predicted survival was 6.3 months, and the best-case scenario was 55.8 months. Accuracy was less for worst-case scenarios. Similar results have been demonstrated for advanced-stage lung cancer.[7]
- Oncologists' survival estimates may serve as a reasonable means to estimate best-case, worst-case, and typical outcomes. Researchers used the responses from oncologists who were asked to estimate expected survival time for each patient with advanced cancer enrolled in a randomized, double-blind, placebo-controlled trial of sertraline.[8] The oncologists were asked to record their estimates as a median time for a group of identical patients. The accuracy of the oncologists' estimates was defined by the proportion of patients with an observed survival time bounded by prespecified multiples of their estimated survival times. The median OS of the cohort of 114 patients was 11 months. Sixty-three percent of the patients had an observed survival ranging from half to double the oncologists' estimates. Although the authors concluded that the oncologists' estimates may serve as the basis for predicting survival, other factors such as the KPS were more predictive of outcome on multivariate analysis.
- The approach is a reasonable way to communicate life expectancy, as judged by people who have cancer. Cancer patients completed a survey after reviewing two formats for explaining life expectancy to a hypothetical patient: either three scenarios (typical, best-case, or worst-case outcomes) or median survival. More respondents agreed that having the three scenarios explained would make sense, be helpful, convey hope, and reassure.[9] For information about their own life expectancies, 88% of respondents preferred being presented with all three scenarios.
Advance Care Planning (ACP) The 1990 Patient Self-Determination Act guarantees patients the right to accept or refuse treatment and to complete advance medical directives to document their EOL preferences or to appoint someone to make decisions on their behalf in the event that their ability to make decisions is compromised. Studies indicate, however, that many patients have not participated in effective ACP. Several reasons might explain the underuse of ACP:[10,11,12] - Initial studies of ACP did not demonstrate the anticipated improvement in outcomes.
- Physicians and patients are ambivalent about ACP.
- Patients' preferences may vary over time.
- There was a failure to transfer information about preferences between inpatient and outpatient or different venues of care.
This section provides a glossary of ACP and highlights the ambivalence that surrounds ACP. It includes a review of subsequent evidence that demonstrates the benefit of ACP, concluding with the suggestion that a more nuanced approach to ACP will improve care near the EOL for patients who have advanced cancer. The language of ACP A brief glossary of the language of ACP may minimize confusion. Documenting ACP decisions Additional documents to communicate the patient's preferences include the following: - Do-not-resuscitate (DNR) order: Tells medical staff in a hospital or nursing facility not to administer cardiopulmonary resuscitation (CPR) if the patient's heart stops beating.
- Out-of-hospital DNR order: Alerts emergency medical personnel to the nonhospitalized patient's wishes regarding CPR and other resuscitation measures. The legal requirements for an out-of-hospital DNR order may differ from state to state but typically involve a document signed by the patient, a witness, and the physician. Patients and informal caregivers are advised to have multiple copies so that the document is immediately available to emergency medical personnel.
- Do-not-intubate (DNI) order: Tells medical staff in the hospital or nursing facility that the patient does not wish to be put on a breathing machine.
- Physician Orders for Life-Sustaining Treatment (POLST): Allows patients to specify the kind of medical treatment they want near the EOL. Printed and signed by both doctor and patient, the POLST document helps give seriously ill patients more control over their EOL care.
- Medical Orders for Life-Sustaining Treatment (MOLST): An initiative that is intended to ensure that patients will receive EOL care according to their wishes and best interests. The MOLST document allows patients to indicate the type of care they would like to receive in situations when they cannot communicate, such as during resuscitation, intubation, and other life-sustaining treatments. Under current law, the information in a MOLST document must be adhered to, in both the home and the hospital, by all medical practitioners, including emergency medical service personnel.
Patient and physician ambivalence about ACP One of the major impediments to effective ACP is patient and physician ambivalence. Patients with advanced cancer may be ambivalent about engaging in ACP with their oncologists because they perceive that oncologists are reluctant to engage in this discussion.[11,13] For example, one group of researchers reported that 87% of women with metastatic breast cancer had discussed EOL decisions with family or friends; 75% had gathered information about advance directives; and 66% had a written advance directive.[14] However, only 19% of those women had discussed EOL decisions with providers; only 24% had shared the written advance directives with the providers; and only 14 % of providers were aware of the advance directives. A study of hospitalized elderly patients similarly found that many had thought about EOL preferences (76.3%) or had an advance care plan (47.9%), but relatively few (30.3%) had discussed them with their physicians.[15] These results suggest that patients engage in ACP but are reluctant to share their thoughts with their oncologists. Nevertheless, the oncologist is in the best position to know when to bring up the subject of EOL care, so he or she can initiate and thoughtfully guide the ACP discussion.[16] ACP Reconsidered Equivocal evidence is a source of physician ambivalence about ACP. Despite early concerns, however, there is growing evidence that ACP improves EOL care in patients with advanced cancer. Early concerns about limits of ACP Initial evidence about ACP was not favorable. For example, several studies found that: - Fewer than 50% of terminally ill patients had advance directives in their medical records.[17,18,19]
- More than 65% of physicians were unaware that their patients had documented advance directives.[20]
- Advance directives were not documented, were difficult to interpret, or were difficult for surrogates and physicians to follow.[17,21]
Potential benefits of ACP Since the 1990s, randomized trials of ACP have demonstrated some benefit. For example, a randomized study compared usual care with a structured approach that included a motivational conversation with a social worker, and a booklet that described ACP and sought information about the patient's desired health states and values.[22] The primary outcomes were comparisons of how closely providers and patients agreed about the following: - Patient preferences to forgo life-prolonging treatment in hypothetical health states.
- Values.
- Personal beliefs about hypothetical health states.
The intervention was associated with increased frequency of ACP discussions between physician and patient (64% vs. 38%; P < .001), and the intervention group had higher levels of agreement for all three outcomes.[22] The effect of ACP on health care outcomes was not studied. A 2010 study monitored 309 patients older than 80 years and their caregivers for 6 months or until a patient died.[23] Researchers found that patients who engaged in ACP were more likely to have preferences that are known to others, and to receive care consistent with those preferences. In addition, compared with family members of patients in the control group, family members of patients in the intervention (with ACP) group who died had significantly less stress (intervention 5, control 15; P < .001), anxiety (intervention 0, control 3; P = .02), and depression (intervention 0, control 5; P = .002). Also, patient and family satisfaction were higher in the intervention group.[23] Studies funded by the Agency for Healthcare Research and Quality show that satisfaction in patients aged 65 years and older increased when patients engaged in ACP with their doctors. Specifically, patients who discussed their preferences for EOL care experienced less anxiety and fear; felt more sense of control over their medical care; and believed that their physicians had a better understanding of their wishes. Surrogates had similar benefits.[24] A 2011 report also suggests that ACP contributes significantly to higher-value EOL care. Researchers demonstrated that the association between evidence of ACP and reduced EOL Medicare expenditures varied according to the level of spending on aggressive treatments near the EOL.[25] Treatment-limiting advance directives were associated with lower levels of Medicare spending, lower likelihood of in-hospital death, and increased use of hospice in high-spending regions but not in low- or medium-spending regions. At a minimum, the study suggests that ACP may be one explanation for lower-cost regions. A more nuanced approach to ACP Advocates maintain that ACP has been too narrowly construed as completion of a document that expresses a patient's EOL preferences or identifies a surrogate decision maker if a patient can no longer make decisions. Generally speaking, most patients from Western countries express greater needs for information related to their illnesses, treatment options, prognoses, future symptoms, likely trajectories of illness, and the dying process. Younger and more educated patients tend to seek more detailed information, while older patients and those from non-Western cultures tend to prefer nondisclosure. From that perspective, the limitations of ACP can be addressed by reconceptualizing ACP. One proposal is to imagine ACP as an ongoing discussion designed to prepare patients and their families or friends to participate more meaningfully in future medical decisions, as required.[26] A focus group study of patients and surrogate decision makers identified four salient themes in discussing future health care decisions:[27] - Identify values based on experiences and QOL.
- Choose surrogates wisely and verify that they understand their roles.
- Decide how much discretion to allow surrogate decision makers.
- Inform others to prevent conflict.
Before discussing ACP, it is essential that the oncologist determine the information needs of the patient so that communication and ACP can be tailored accordingly.[28] Interventions to Support Patient-Oncologist Communication Various interventions to support patient-oncologist communication have been studied. There are few comparative trials, and the outcomes of interest have varied, so it is not possible to evaluate the interventions. However, the variety of interventions will allow the interested oncology clinician to find one of interest. Written information Myriad informational booklets are available for patients to consult; prognostic information is discussed in some of these booklets. Researchers conducted a mixed-methods study of the views of cancer patients on written materials provided by their oncologists.[29] Patients expressed concerns that conflicting information could undermine their trust in their oncologists, and this concern influenced their willingness to consult written materials. Another caution was raised by a randomized study that compared QOL and satisfaction between lung cancer patients scheduled for surgery who received verbal information only and those who received verbal and written information.[30] There were no differences in patient satisfaction with information; however, patients who received verbal and written information were less satisfied with the hospital stay. Thus, the presentation of written information may have unintended consequences, and oncologists may consider distributing only information that reflects their actual practices. Audiotaped consultations The impact of simply recording consultations on audiotape is significant, especially given the ease of doing so. In one randomized trial of initial consultations for all stages of cancer, 105 patients received audiotapes and 96 did not.[31] Patients who received the audiotapes were more satisfied and had better recall of information than did those who did not receive the tapes. In addition, younger patients were more satisfied, and older patients experienced enhanced recall. Another study reported similar results.[32] Video-recorded information The use of videos to facilitate discussions between providers, patients, and families and to assist with informed decision making has been demonstrated to be effective in at least two randomized controlled trials.[33,34] In both studies, patients and family members (where appropriate) found the videos acceptable and were comfortable with their content. In the first study, 50 patients with malignant glioma were randomly assigned to hear a verbal narrative or to hear the same verbal narrative and be shown a video that described three types of care:[33] - Life-prolonging care (extending life at any cost).
- Basic medical care (including most care, but excluding CPR, intubation, mechanical ventilation, and admission to an intensive care unit).
- Comfort care (relieving symptoms, maximizing comfort, and generally avoiding hospitalization).
Participants in the sample had a mean age of 54 years; 44% were female, 50% did not want CPR at baseline, and 76% had advance directives. When asked postintervention about their preferences for type of care, 26% of participants in the verbal control group preferred life-prolonging care, compared with none in the video intervention group. More patients in the video intervention group preferred comfort care than did patients in the verbal control group (91% vs. 22%, respectively).[33] In the second study, 150 patients with advanced cancer from four cancer centers were randomly assigned to listen to a verbal description of CPR or to listen to the same verbal description and then be shown a 3-minute video of simulated CPR being done on a patient on a ventilator.[34] The primary outcome was participants' preference for or against CPR measured immediately after exposure to either modality. As in the first study, more patients receiving the video intervention with the narrative decided against CPR than did those receiving only the narrative (79% vs. 51%, respectively). Knowledge scores were also significantly higher in the video arm (P < .001). Results at the 6- to 8-week follow-up demonstrated fairly stable decision results. These studies suggest that videos can be used as an adjunct to oncologist-patient communication and education. Question prompt lists Patients may have difficulty posing questions about sensitive and difficult issues such as prognosis or EOL care. Question prompt lists can be prepared to help guide patients through such questions. One group of researchers developed and tested a question prompt list for advanced cancer patients to use during consultation with a palliative care physician.[35] Patients randomly assigned to the intervention arm more frequently asked more questions related to prognosis and EOL issues than did patients in the control group (30% vs. 10%; P = .001). There were no negative effects on patient satisfaction or anxiety. A slightly different strategy-addressing the prompt list to informal caregivers of patients with advanced cancer-was evaluated in another study and appeared promising.[36] Cancer consultation preparation package (CCPP) A CCPP developed by one group of researchers consists of four components:[37] - A booklet that describes the cancer center's procedures and layout.
- A booklet that introduces the concept of evidence-based decision making and factors other than evidence that influence decisions.
- A booklet that describes the patient's rights and responsibilities.
- A question prompt list.
A randomized trial of the CCPP versus a control booklet in 164 initial consultations for patients with a variety of cancers at different stages demonstrated that patients receiving the CCPP asked significantly more questions (11 questions vs. 7 questions; P = .005) without increasing their anxiety. However, patients assigned to the CCPP did not participate in decision making more often or more actively than did patients in the control group.[37] Pamphlet and psychologist-facilitated discussion In a randomized trial, an ACP pamphlet supplemented by a discussion with a psychologist was compared with usual care.[38] Patients in both cohorts had equivalent rates of DNR orders (68% vs. 76%), but DNR orders for the intervention group were placed at a median of 27 days, compared with 12.5 days for the control group (P = .03). Intervention patients were less likely to die in the hospital (19% vs. 50%), and there was no increased sense of anxiety. Interpretation of the study results is hampered by a high rate of loss to follow-up, and the intention-to-treat analysis was not significant. Decision Aids Decision aids are more complicated interventions designed to provide patients with a balanced summary of information about potential treatment options and possible outcomes, to allow patients to make informed decisions consistent with their preferences. Decision aids complement the patient-oncologist relationship and have been studied in two situations relevant to the care of patients with advanced cancer: the decision to undergo chemotherapy in advanced cancer and the decision to prepare for the EOL. The decision to undergo chemotherapy In a 2011 study of patients with metastatic colorectal cancer who were deciding whether to initiate first-line systemic chemotherapy, 207 patients were randomly assigned to receive either a standard oncology consultation or a standard consultation supplemented by a take-home booklet and audio recording of the consultation.[39] Patients assigned to the decision aid demonstrated increased understanding of the prognosis, treatment options, and goals of treatment. There were no differences in decisional conflict, levels of anxiety, patient achievement of preferred level of involvement in decision making, or treatment selected. The decision to engage in ACP In a pilot semirandomized trial, patients with advanced cancer who met with a trained care planning mediator were more likely to discuss future preferences with families and friends (but not with their oncologists).[40] Slightly more than half of patients with advanced cancer were willing to meet with an independent trained counselor about ACP. Patients assigned to meet with the mediator tended to be less satisfied with communication with health care professionals and less satisfied with future care. Thus, while ACP with a trained mediator is feasible, the costs of additional personnel may be prohibitive and create unmet expectations in patients. Decisions related to resuscitation status Several approaches to facilitate discussion about a patient's resuscitation preferences and status have been studied. The approaches include the following: - The use of computer-based prompts to advise the clinician to initiate the discussion.[41]
- An informational DVD and booklet for family caregivers to better understand the resuscitation option.[34]
- Audiovisual vignettes to illustrate the process and outcomes of resuscitation.[42]
- A structured discussion with a clinical psychologist.[38]
In several studies, DNR orders were more common in the intervention group or occurred earlier. The secondary outcomes included earlier and higher rates of discussions with patients and family members regarding resuscitation, lower decisional conflict and uncertainty, and more value clarification. In at least two studies, there was no increase in anxiety and/or depressive symptoms. These decision aids are limited to use among adult patients. Physician attitudes toward decision aids Researchers conducted a cross-sectional survey of Canadian medical, radiation, and surgical oncologists to determine their levels of awareness and utilization of patient decision aids.[43] Slightly fewer than half (46%) were aware of decision aids relevant to their practices, and only 24% utilized aids in their practices. Most respondents recognized the important clinical outcomes associated with decision aids, i.e., increased knowledge, reduced anxiety, and increased patient satisfaction. Lack of awareness was the most frequently cited barrier to use (69%), followed by lack of resources (24%) and lack of time to learn about decision aids (24%). Only 3% of respondents cited lack of time as a barrier to the use of decision aids. Strategies to introduce decision aids into clinical practice are clearly needed and could start with educating oncologists about available decision aids. Integrating Palliative Care and Conventional Cancer Care In 2012, the American Society of Clinical Oncology published a provisional clinical opinion advising its membership that "… combined standard oncology care and palliative care should be considered early in the course of illness for any patient with metastatic cancer and/or high symptom burden."[44] The authors primarily based their recommendation on two randomized clinical trials of palliative care interventions during conventional cancer care for people with metastatic cancer that supported results of several older and inconclusive randomized clinical trials.[45] Subsequently, two additional randomized trials comparing outcomes between cohorts of patients who received conventional oncology care and a concurrent palliative care intervention or conventional oncology care at different times have been reported.[46,47] The four studies are summarized below: ENABLE II: A randomized trial of an advance practice nurse-led psychoeducational intervention This group of investigators reported the results of Project ENABLE (Educate, Nurture, Advise Before Life Ends).[48] Three hundred twenty-two patients with advanced cancer were randomly assigned to receive either usual care or a multicomponent psychoeducational intervention conducted by advance practice nurses concurrent with usual care. The primary outcomes were QOL, as measured by the Functional Assessment of Chronic Illness Therapy for Palliative Care; symptom intensity, as measured by the Edmonton Symptom Assessment Scale; and mood, as measured by the Center for Epidemiological Studies Depression Scale. The intervention was based on the chronic care model; the goal was to encourage patient involvement in care planning, self-management, and empowerment. The intervention consisted of four weekly educational sessions and a monthly follow-up. The four modules consisted of the following: - Problem solving.
- Communication and social support.
- Symptom management.
- ACP (advance care planning) and unfinished business.
The mean scores for the participants in the intervention group were 4.6 higher for QOL (P = .02); 27.8 lower for symptom intensity (P = .06); and 1.8 lower for depressed mood (P = .02). Patients who died during the study had less-marked treatment effects. There was no difference in OS or use of chemotherapy or aggressive medical care near the EOL. Furthermore, there were no differences between groups in rates of referral to palliative care or hospice.[48] Randomized trial of concurrent palliative care and standard oncology care for patients with metastatic lung cancer The second study randomly assigned 151 patients with newly diagnosed metastatic NSCLC to receive either standard oncology care or standard oncology care with the early integration of palliative care. Patients assigned to palliative care met with a member of the palliative care team within 3 weeks of diagnosis. General guidelines called for palliative care clinicians to pay specific attention to the following: - Assessment of physical and psychological symptoms.
- Establishment of goals of care.
- Assistance with decision making about treatment.
- Coordination of care on the basis of the patient's individual needs.
The primary and secondary analyses of the study have been reported in an original publication and then in several secondary publications,[49,50,51] summarized below. Cluster-randomized trial of early palliative care for patients with advanced cancer This single-blinded study reported the outcomes of patients who were assigned to receive palliative care and conventional cancer care or conventional cancer care alone.[55] The palliative care intervention included an initial visit followed by monthly palliative care clinic visits, with telephone follow-up and direct access to the inpatient palliative care unit as needed. The primary outcome was the change score for the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp) at 3 months. Secondary outcomes were the FACIT-Sp at 4 months and other validated measures of QOL, symptom burden, satisfaction with care, and quality of medical interactions at 3 and 4 months. There were no statistically significant differences in the change FACIT-Sp scores at 3 months, but differences did emerge at 4 months; in addition, there were significant differences in the secondary outcomes at 3 and 4 months. These results confirm the benefit of concurrent palliative care and suggest that the benefits of palliative care will increase over time. The coordination of care between the Princess Margaret Cancer Centre and Community Care Access Centres may make the findings difficult to replicate.[55] ENABLE III: Early versus delayed initiation of concurrent palliative oncology care This study compared the outcomes of 207 patients randomly assigned to begin receiving palliative care interventions within 30 to 60 days of diagnosis versus 3 months from diagnosis of advanced cancer.[47] The weekly telephone coaching sessions described in the summary of ENABLE II included a three-session life review component and was designed to specifically intervene with caregivers. The outcomes of interest were QOL, symptom impact, mood, resource use, and improved 1-year survival. Early palliative oncology care was associated with an improved survival (63% vs. 48%, P = .038). There were, however, no statistically significant differences in patient-reported outcomes or resource use. Furthermore, the OS log-rank test did not reveal a significant difference, suggesting the survival curves converge after the 1-year point. A few limitations of the study potentially complicate interpretation. First, the study did not meet its accrual goal of 360 patients, in part because of high rates of ineligibility after screening and patient refusal. Second, the intention-to-treat analysis may have missed differences due to variations in the "amount" of the intervention completed. Third, the multiple outcomes may have limited the statistical power to detect meaningful differences, especially given the smaller-than-desired sample size. Thus, a more-accurate interpretation of the results may be that the optimal time of palliative care interventions remains undetermined. In contrast to the results for patients, the ENABLE III intervention improved outcomes for caregivers.[56] One-hundred twenty-two caregivers were randomly assigned to receive, either early or at a later time, three weekly telephone coaching sessions, monthly follow-up, and a bereavement call. For all caregivers, the early intervention was associated with lower depression scores at 3 months. Caregivers of decedents who received palliative care early had lower depression and burden in the terminal phase. These results are notable in establishing a benefit to caregivers. Challenges to the early integration of palliative care Several of the more relevant challenges to the integration of palliative care into standard oncology care are listed below, followed by a summary of the available data. - Availability of specialty palliative care services: In a survey published in 2010, only 59% of National Cancer Institute (NCI) cancer centers and 33% of non-NCI cancer centers reported having palliative care programs, with the level of resources for programs varying widely.[57] However, at least half of the executives surveyed indicated an interest in expanding services in the coming years, although there were differences based on the NCI designation. Of interest is that the identified impediments to developing services further were more related to poor reimbursement or limited institutional budget than to concerns about the evidence supporting the services.
One potential solution to the workforce shortage is to train oncologists in the skills of so-called primary palliative care.[58] The skills of primary palliative care include basic discussions about prognosis, goals of treatment, suffering, or code status and symptom management, thus reserving specialty referrals for assistance with conflict resolution regarding goals or methods of treatment. - Oncologists' referral of patients to specialty palliative care services: A survey of Canadian cancer specialists demonstrated that 94% had access to at least one component of palliative care services, but only 36% of respondents had access to comprehensive palliative care services.[59] In addition, 80% of physicians indicated that they always or usually referred terminally ill patients, with the time of referral evenly split between at diagnosis, during palliative chemotherapy, or after chemotherapy or transfusions were stopped.
- Oncologist attitudes toward palliative care.
- Patient acceptance.
- Unanswered questions: The results of the four randomized trials summarized above provide a compelling rationale for providing access to palliative care services concurrent with conventional cancer care.[60,61] However, there are unanswered questions that might diminish enthusiasm for the systematic integration of palliative care into conventional cancer care.
- Who are the patients to be served? A review of the eligibility criteria demonstrates that patients were chosen on the basis of characteristics in addition to the disease stage, such as physician-estimated patient survival. As indicated in Table 1, many potentially eligible patients declined to participate in the trials. The various reasons for their nonparticipation require clarification to identify potential confounders that may affect the likelihood of benefit from services.
- When is the optimal time to introduce specialty palliative care?
- Which components of palliative care are critical for positive outcomes? The ENABLE interventions included multiple educational sessions led by an advanced practice nurse over the course of months. In addition, there was only a single scheduled palliative care consultation at study initiation. This suggests that alternatives to the multidisciplinary palliative care teams described by other investigators [46,50] may be impactful.
- What is the mechanism of action of the putative benefit? This question seems especially critical because cancer centers struggle with declining reimbursements, so there is a legitimate need to optimize the value of the supportive services provided.
- What are the mechanisms that explain the observed improvement in survival?[48,62,63]
- Funding: A survey of 20 outpatient palliative care clinics indicated that billing for services and institutional support were the primary sources of funding, but most identified funding for staff as a major challenge.[64]
Table 1. Characteristics of Subjects in Randomized Trials of Concurrent Palliative Care in Advanced Cancer| Author/Reference | Recruitment (No.) | Selected Eligibility Criteria |
|---|
| Randomized | Potentially Eligible | Screened | Type of Cancer | Time from Diagnosis | Oncologist-Estimated Survival |
|---|
| GI = gastrointestinal; GU = genitourinary, hem = hematologic. | | Bakitas et al., 2009[48] | 322 | 681 | 1,222 | GI; lung; GU; breast | Within 8-12 weeks | ~1 year | | Temel et al., 2010[49] | 151 | | | Lung | ≤8 weeks | Not specified | | Zimmermann et al., 2014[46] | 461 | 892 | 3,293 | GI; lung; GU; breast; gynecologic | Not specified | 6-24 months | | Bakitas et al., 2015[47] | 207 | 545 | 1,468 | GI; lung; GU; breast; other; hem malignancy | Early: 30-60 days; delayed: >90 days | 6-24 months | Table 2. Endpoints and Measures from Randomized Trials of Concurrent Palliative Care in Advanced Cancer| Endpoint | Measure |
|---|
| CARES-MIS = Cancer Rehabilitation Evaluation System Medical Interaction Subscale; CES-D = Center for Epidemiologic Studies Depression Scale; ED = emergency department; ENABLE = Educate, Nurture, Advise Before Life Ends; ESAS = Edmonton Symptom Assessment System; FACIT-Pal = Functional Assessment of Chronic Illness Therapy - Palliative Care; FACIT-Sp = Functional Assessment of Chronic Illness Therapy - Spiritual Well-Being; FACT-L = Functional Assessment of Cancer Therapy - Lung; FAMCARE-P16 = 16-item measure of patient satisfaction with cancer care; HADS = Hospital Anxiety and Depression Scale; ICU = intensive care unit; NR = outcome measured but not reported; QUAL-E = Quality of Life at the End of Life; - = outcome not measured. | | | Bakitas et al., 2009 (ENABLE II)[48] | Temel et al., 2010[49] | Zimmermann et al., 2014[46] | Bakitas et al., 2015 (ENABLE III)[47] | | Quality of life | FACIT-Pal | FACT-L | FACIT-Sp; QUAL-E | FACIT-Pal; QUAL-E | | Mood | CES-D | HADS | - | - | | Symptoms | ESAS | - | QUAL-E | ESAS | | Satisfaction with care | - | - | FAMCARE-P16 | - | | Medical interactions | - | - | CARES-MIS | - | | Chemotherapy use | NR | NR | NR | NR | | End-of-life resource use | Hospital/ICU days; ED visits | Hospital/ICU days; ED visits; chemotherapy within 14 days of death; hospice utilization; place of death | Not specified | Hospital/ICU days; ED visits; chemotherapy within 14 days of death; place of death | | Median overall survival | Yes | Yes | - | Yes | Table 3. Studies on the Timing of Oncology Care and Palliative Care| Outcome | Author/Reference |
|---|
| Bakitas et al., 2009 (ENABLE II)[48] | Temel et al., 2010[49] | Zimmermann et al., 2014[46] | Bakitas et al., 2015 (ENABLE III)[47] |
|---|
| ENABLE = Educate, Nurture, Advise Before Life Ends; NR = outcome measured but not reported; NS = no significant difference in outcomes detected; PC = favors palliative care intervention; - = outcome not measured. | | Quality of life | PC | PC | NS at 3 months; PC at 4 months | NS | | Mood | PC | PC | NS at 3 months; PC at 4 months | NS | | Symptoms | NS | - | NS at 3 months; PC at 4 months | NS | | Satisfaction with care | - | - | PC at 3 months; PC at 4 months | - | | Medical interactions | - | - | NS at 3 months; NS at 4 months | - | | End-of-life resource use | NS | NR | NR | NS | | Overall survival | NS | PC | NR | Early | References:
-
Krishnan M, Temel JS, Wright AA, et al.: Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol 11 (2): 68-74, 2013.
-
Movsas B, Moughan J, Sarna L, et al.: Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol 27 (34): 5816-22, 2009.
-
Sloan JA, Zhao X, Novotny PJ, et al.: Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. J Clin Oncol 30 (13): 1498-504, 2012.
-
Hagerty RG, Butow PN, Ellis PA, et al.: Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 22 (9): 1721-30, 2004.
-
Stockler MR, Tattersall MH, Boyer MJ, et al.: Disarming the guarded prognosis: predicting survival in newly referred patients with incurable cancer. Br J Cancer 94 (2): 208-12, 2006.
-
Kiely BE, Soon YY, Tattersall MH, et al.: How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol 29 (4): 456-63, 2011.
-
Kiely BE, Alam M, Blinman P, et al.: Estimating typical, best-case and worst-case life expectancy scenarios for patients starting chemotherapy for advanced non-small-cell lung cancer: a systematic review of contemporary randomized trials. Lung Cancer 77 (3): 537-44, 2012.
-
Kiely BE, Martin AJ, Tattersall MH, et al.: The median informs the message: accuracy of individualized scenarios for survival time based on oncologists' estimates. J Clin Oncol 31 (28): 3565-71, 2013.
-
Kiely BE, McCaughan G, Christodoulou S, et al.: Using scenarios to explain life expectancy in advanced cancer: attitudes of people with a cancer experience. Support Care Cancer 21 (2): 369-76, 2013.
-
Teno JM: Advance directives: time to move on. Ann Intern Med 141 (2): 159-60, 2004.
-
Barnes KA, Barlow CA, Harrington J, et al.: Advance care planning discussions in advanced cancer: analysis of dialogues between patients and care planning mediators. Palliat Support Care 9 (1): 73-9, 2011.
-
Hawkins NA, Ditto PH, Danks JH, et al.: Micromanaging death: process preferences, values, and goals in end-of-life medical decision making. Gerontologist 45 (1): 107-17, 2005.
-
Dow LA, Matsuyama RK, Ramakrishnan V, et al.: Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol 28 (2): 299-304, 2010.
-
Ozanne EM, Partridge A, Moy B, et al.: Doctor-patient communication about advance directives in metastatic breast cancer. J Palliat Med 12 (6): 547-53, 2009.
-
Heyland DK, Barwich D, Pichora D, et al.: Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med 173 (9): 778-87, 2013.
-
Emanuel LL, Danis M, Pearlman RA, et al.: Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc 43 (4): 440-6, 1995.
-
Teno JM, Licks S, Lynn J, et al.: Do advance directives provide instructions that direct care? SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc 45 (4): 508-12, 1997.
-
Teno J, Lynn J, Wenger N, et al.: Advance directives for seriously ill hospitalized patients: effectiveness with the patient self-determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc 45 (4): 500-7, 1997.
-
Bradley EH, Rizzo JA: Public information and private search: evaluating the Patient Self-Determination Act. J Health Polit Policy Law 24 (2): 239-73, 1999.
-
Virmani J, Schneiderman LJ, Kaplan RM: Relationship of advance directives to physician-patient communication. Arch Intern Med 154 (8): 909-13, 1994.
-
Teno JM, Lynn J, Phillips RS, et al.: Do formal advance directives affect resuscitation decisions and the use of resources for seriously ill patients? SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Clin Ethics 5 (1): 23-30, 1994.
-
Pearlman RA, Starks H, Cain KC, et al.: Improvements in advance care planning in the Veterans Affairs System: results of a multifaceted intervention. Arch Intern Med 165 (6): 667-74, 2005.
-
Detering KM, Hancock AD, Reade MC, et al.: The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 340: c1345, 2010.
-
Kass-Bartelmes BL, Hughes R: Advance Care Planning, Preferences for Care at the End of Life: Research in Action, Issue 12. Rockville, Md: Agency for Healthcare Research and Quality, 2003. Available online. Last accessed February 27, 2017.
-
Nicholas LH, Langa KM, Iwashyna TJ, et al.: Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA 306 (13): 1447-53, 2011.
-
Sudore RL, Fried TR: Redefining the "planning" in advance care planning: preparing for end-of-life decision making. Ann Intern Med 153 (4): 256-61, 2010.
-
McMahan RD, Knight SJ, Fried TR, et al.: Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage 46 (3): 355-65, 2013.
-
Johnston G, Abraham C: Managing awareness: negotiating and coping with a terminal prognosis. Int J Palliat Nurs 6 (10): 485-94, 2000 Nov-Dec.
-
Davey HM, Armstrong BK, Butow PN: An exploratory study of cancer patients' views on doctor-provided and independent written prognostic information. Patient Educ Couns 56 (3): 349-55, 2005.
-
Barlési F, Barrau K, Loundou A, et al.: Impact of information on quality of life and satisfaction of non-small cell lung cancer patients: a randomized study of standardized versus individualized information before thoracic surgery. J Thorac Oncol 3 (10): 1146-52, 2008.
-
Ong LM, Visser MR, Lammes FB, et al.: Effect of providing cancer patients with the audiotaped initial consultation on satisfaction, recall, and quality of life: a randomized, double-blind study. J Clin Oncol 18 (16): 3052-60, 2000.
-
Bruera E, Pituskin E, Calder K, et al.: The addition of an audiocassette recording of a consultation to written recommendations for patients with advanced cancer: A randomized, controlled trial. Cancer 86 (11): 2420-5, 1999.
-
El-Jawahri A, Podgurski LM, Eichler AF, et al.: Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol 28 (2): 305-10, 2010.
-
Volandes AE, Paasche-Orlow MK, Mitchell SL, et al.: Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. J Clin Oncol 31 (3): 380-6, 2013.
-
Clayton JM, Butow PN, Tattersall MH, et al.: Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 25 (6): 715-23, 2007.
-
Hebert RS, Schulz R, Copeland VC, et al.: Pilot testing of a question prompt sheet to encourage family caregivers of cancer patients and physicians to discuss end-of-life issues. Am J Hosp Palliat Care 26 (1): 24-32, 2009 Feb-Mar.
-
Butow P, Devine R, Boyer M, et al.: Cancer consultation preparation package: changing patients but not physicians is not enough. J Clin Oncol 22 (21): 4401-9, 2004.
-
Stein RA, Sharpe L, Bell ML, et al.: Randomized controlled trial of a structured intervention to facilitate end-of-life decision making in patients with advanced cancer. J Clin Oncol 31 (27): 3403-10, 2013.
-
Leighl NB, Shepherd HL, Butow PN, et al.: Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol 29 (15): 2077-84, 2011.
-
Jones L, Harrington J, Barlow CA, et al.: Advance care planning in advanced cancer: can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Palliat Support Care 9 (1): 3-13, 2011.
-
Temel JS, Greer JA, Gallagher ER, et al.: Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol 31 (6): 710-5, 2013.
-
Yun YH, Lee MK, Park S, et al.: Use of a decision aid to help caregivers discuss terminal disease status with a family member with cancer: a randomized controlled trial. J Clin Oncol 29 (36): 4811-9, 2011.
-
Brace C, Schmocker S, Huang H, et al.: Physicians' awareness and attitudes toward decision aids for patients with cancer. J Clin Oncol 28 (13): 2286-92, 2010.
-
Smith TJ, Temin S, Alesi ER, et al.: American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 30 (8): 880-7, 2012.
-
Lorenz KA, Lynn J, Dy SM, et al.: Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 148 (2): 147-59, 2008.
-
Zimmermann C, Swami N, Krzyzanowska M, et al.: Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 383 (9930): 1721-30, 2014.
-
Bakitas MA, Tosteson TD, Li Z, et al.: Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 33 (13): 1438-45, 2015.
-
Bakitas M, Lyons KD, Hegel MT, et al.: Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 302 (7): 741-9, 2009.
-
Temel JS, Greer JA, Muzikansky A, et al.: Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363 (8): 733-42, 2010.
-
Temel JS, Greer JA, Admane S, et al.: Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 29 (17): 2319-26, 2011.
-
Greer JA, Pirl WF, Jackson VA, et al.: Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 30 (4): 394-400, 2012.
-
Pirl WF, Greer JA, Traeger L, et al.: Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol 30 (12): 1310-5, 2012.
-
Jacobsen J, Jackson V, Dahlin C, et al.: Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 14 (4): 459-64, 2011.
-
Yoong J, Park ER, Greer JA, et al.: Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med 173 (4): 283-90, 2013.
-
Hannon B, Swami N, Pope A, et al.: The oncology palliative care clinic at the Princess Margaret Cancer Centre: an early intervention model for patients with advanced cancer. Support Care Cancer 23 (4): 1073-80, 2015.
-
Dionne-Odom JN, Azuero A, Lyons KD, et al.: Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients With Advanced Cancer: Outcomes From the ENABLE III Randomized Controlled Trial. J Clin Oncol 33 (13): 1446-52, 2015.
-
Hui D, Elsayem A, De la Cruz M, et al.: Availability and integration of palliative care at US cancer centers. JAMA 303 (11): 1054-61, 2010.
-
Quill TE, Abernethy AP: Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med 368 (13): 1173-5, 2013.
-
Wentlandt K, Krzyzanowska MK, Swami N, et al.: Referral practices of oncologists to specialized palliative care. J Clin Oncol 30 (35): 4380-6, 2012.
-
Parikh RB, Kirch RA, Smith TJ, et al.: Early specialty palliative care--translating data in oncology into practice. N Engl J Med 369 (24): 2347-51, 2013.
-
Gomes B: Palliative care: if it makes a difference, why wait? J Clin Oncol 33 (13): 1420-1, 2015.
-
Temel JS, Jackson VA, Billings JA, et al.: Phase II study: integrated palliative care in newly diagnosed advanced non-small-cell lung cancer patients. J Clin Oncol 25 (17): 2377-82, 2007.
-
Gaertner J, Wolf J, Hallek M, et al.: Standardizing integration of palliative care into comprehensive cancer therapy--a disease specific approach. Support Care Cancer 19 (7): 1037-43, 2011.
-
Smith AK, Thai JN, Bakitas MA, et al.: The diverse landscape of palliative care clinics. J Palliat Med 16 (6): 661-8, 2013.
The Oncology Clinician's Role in Planning the Transition to End-of-Life CarePerspective In 1961, a survey of 219 physicians was published, with 88% of respondents indicating that their "usual policy about telling patients" they have cancer was "don't tell"; however, 34% of the group indicated that they occasionally made exceptions and disclosed the diagnosis, most often to family members.[1] The identical survey was repeated in 1977, with a different group of 264 physicians; at that time, 98% of respondents indicated that telling a patient about the diagnosis of cancer was their usual policy, although 28% reported occasionally making an exception.[2] The effective communication of prognosis remains a challenge, however, as measured by patient-reported estimates of life expectancy or goals of treatment. For example, in 1984, one study reported that 37% of patients with metastatic, incurable cancer felt that treatment would cure their disease.[3] Similarly, another study reported in 2012 that 69% of patients with advanced lung cancer and 81% of patients with advanced colorectal cancer enrolled in the Cancer Care Outcomes Research and Surveillance (CanCORS) study were unaware that chemotherapy was not likely to cure their disease.[4] In fact, almost 40% of patients with colorectal cancer responded that chemotherapy was very likely to cure the disease. A related analysis of the CanCORS data set revealed that only 16.5% of patients reported an accurate awareness of their prognosis.[5] Similar results have been reported from a study of patients with advanced cancer that assessed their understanding of their prognosis at enrollment and 12 weeks later.[6] Potential explanations for the communication deficits regarding prognosis are summarized in the Potential Barriers to Planning the Transition to End-of-Life Care section of this summary. Regardless of the explanation, however, misunderstandings about prognosis compromise a patient's ability to make informed health care decisions consistent with his or her values, goals, and preferences. Ultimately, each oncology clinician chooses, on some level, when and how to explain to a patient with advanced cancer that disease-directed treatments are very unlikely to prove effective and a continued focus on treatment risks harming the patient. The goal of this summary section is to provide clinicians with frameworks for considering their role in planning the transition to end-of-life (EOL) care and discussing the concept of transition with patients and their loved ones. The Patient-Clinician Relationship and Planning the Transition to EOL Care It is important for oncology clinicians to consider how their relationship with patients will help patients express preferences that are consistent with their values and goals for receiving high-quality EOL care. One study proposed the following four models of the physician-patient relationship, based on the goals of the interaction, the physician's obligations, the role of patient values, and how patient autonomy influences the relationship:[7] - Paternalistic: The focus is on the physician's assessment and judgment of best treatment, with limited or no patient participation, other than providing consent.
- Informative: The focus is on the physician providing the patient with disease and treatment information that is as complete as possible, with decision making left to the patient.
- Interpretive: The physician acts as a counselor to help the patient discern his or her values and preferences, provides medical information, and then advises the patient about which appropriate tests and treatments best reflect the patient's values.
- Deliberative: The physician's role is to assist the patient in both determining and then choosing the best health-related goals and options, with the attitude of a friend or teacher. The physician plays an active part in suggesting which values and options are the most worthy.
In a carefully reasoned argument, the authors concluded that the preferred choice is the deliberative model, which can be paraphrased as a belief that patients' goals and preferences are open to development and evolution. The goal of this relationship is to help the patient choose the best health-related goals and options that can be achieved, given the clinical situation. The physician's obligation is to articulate for the patient the most compelling goals and preferences and to inform the patient of other options. In that sense, the physician is a teacher.[7] Qualitative studies of patients' perception of decision making suggest that the deliberative clinician-patient relationship is especially critical in advanced-stage cancer. For example, one group of investigators interviewed 36 patients with potentially curable esophageal cancer about their decision to undergo esophagectomy. The investigators anticipated that themes related to autonomy, shared decision making, and information disclosure would become apparent; however, the following themes emerged:[8] - Cultural belief in surgical cure.
- Enhancement of trust through the referral process.
- Idealization of the specialist surgeon.
- Belief in expertise rather than medical information.
- Resignation to the risks of treatment.
- Acceptance of an expert recommendation as consent to treatment.
Another group of investigators interviewed patients with pancreatic carcinoma and identified a change in attitudes toward treatment decision making over time.[9] Initially, patients described little interest in details about medical or surgical treatments and emphasized their trust in the physician. Later, as patients had more experience with disease progression and treatment, they described seeking a more proactive role in treatment decisions. All patients reported discussions about their poor prognosis, but a common theme was the difficulty in recognizing when it was time to stop treatment and transition to EOL care. Planning the Transition from Diagnosis to EOL Care The word transition implies a passage from one place to another. Planning the transition to EOL care, therefore, requires a shared understanding of where the patient is in the advanced-cancer disease trajectory and why a transition is necessary or advisable. The appropriate time to transition to EOL care is when the change is most consistent with the patient's goals of care. In answering the question of when to transition to EOL care, the oncology clinician strives to be a teacher and trusted friend. The clinician must present his or her opinion of the effectiveness of continued cancer-directed treatments and help the patient understand the patient's own values, perspectives, goals of care, and preferences. In this way, the clinician aids the patient in constructing treatment and care preferences toward the EOL.[10] The concept of transition is often invoked from the health care provider's perspective.[11,12] For example, patients who received adjuvant chemotherapy with curative intent are often spoken of as making a transition from the curative to the palliative phase of treatment. The figure below depicts a phase model of planning the transition to EOL care for patients with advanced cancer.
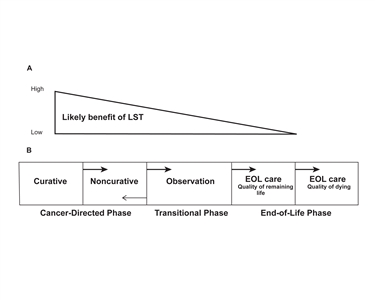
A phase model of planning the transition to end-of-life (EOL) care in advanced cancer. The trajectory of advanced cancer begins with the realization that disease-directed treatments are no longer curative. Over time, the patient, family, and oncology clinician should strive to develop a shared understanding of available treatments and recognize when the likelihood of benefit decreases in relation to the potential harm of treatment. LST = life-sustaining treatment; EOL = end-of-life Panel A of the figure demonstrates the increasingly unfavorable risk-benefit ratio of disease-directed treatments over time. This conceptualization is also from the oncology clinician's perspective. The oncology clinician could choose two tactics to develop a shared understanding of the decline in the risk-to-benefit ratio: - Distinguish between effectiveness and benefit: The health care decisions of patients with advanced cancer toward the EOL may, at times, seem contrary to their best interests from the clinician's perspective.[13] A relevant explanation for the difference may be that patients think in terms of benefit, which includes both the chance of an outcome and its desirability; while clinicians think in terms of the evidence of effectiveness, which is assessed with probabilities.[14]
- Explore patient's understanding of their prognosis and their goals at sentinel events: There are relatively discrete points in the disease trajectory, called sentinel events, when the oncologist has an opportunity to clarify prognosis and discern patient goals and preferences.[15] Sentinel events include scheduled assessments of the disease status (e.g., repeat tumor measurements after a prespecified number of cycles of chemotherapy), effects of chemotherapy on overall health-related quality of life, and the side effects of or complications from disease progression or therapy (e.g., decline in performance status).
Panel B of the figure represents the concept of transitions. The disease trajectory is divided into five relatively discrete phases, from the diagnosis of advanced cancer to death. The first two phases are dominated by disease-directed strategies, including a phase in which the intent of disease-directed treatment is cure; and a second phase in which the intent of treatment is symptom relief, disease control and, perhaps, improved life expectancy. The EOL phase is subdivided into a time in which the patient is not receiving therapy but may still anticipate treatment in the future, and a phase in which the goal of care is to ensure that the EOL is free from undue burden or distress. Similar figures are often modified to include a diagonal line depicting a gradual increase in the focus on palliative care as the cancer progresses and life is more likely to end. The figure represents the advanced-cancer trajectory to help oncologists formulate and articulate the potentially salient differences between the phases from the oncologist's perspective in terms of the following: - Treatment options, intent, range of possible outcomes, and likelihood of potential outcomes.
- The likely experience, including potential risks and the burden of each option in terms of side effects and practical requirements of treatment.
- Key activities (e.g., chemotherapy), scheduled assessments of disease status, and the treatment experience associated with each option or phase.
The oncology clinician should then endeavor to communicate the information in a compassionate manner that is comprehensible to the patient and family. (Refer to the Strategies to Improve Patient-Oncologist Communication and Decision Making in Advanced Cancer section of this summary for more information.) Clearly describing each phase, however, does not necessarily inform patients of when it is time to transition. A focus group study of health care providers, patients with advanced cancer and other terminal illnesses, and family members prioritized three discrete communication skills as valuable to the dying person: giving bad news sensitively, talking about dying, and knowing when the patient was ready to talk about dying.[16] In conclusion, the right time to transition away from disease-directed treatments toward EOL care depends, in large part, on the patient's goals and preferences. The evidence indicates that conversations about EOL care are difficult but of great benefit to the patient. Oncologists who delay initiating these important conversations or who communicate in ambiguous or difficult-to-understand language fail to meet "the physician's central task in caring for a gravely ill person near death[, which] is to accompany and guide the patient, who as a rule does not want to be dead, through the critical transition."[17] References:
-
OKEN D: What to tell cancer patients. A study of medical attitudes. JAMA 175: 1120-8, 1961.
-
Novack DH, Plumer R, Smith RL, et al.: Changes in physicians' attitudes toward telling the cancer patient. JAMA 241 (9): 897-900, 1979.
-
Eidinger RN, Schapira DV: Cancer patients' insight into their treatment, prognosis, and unconventional therapies. Cancer 53 (12): 2736-40, 1984.
-
Weeks JC, Catalano PJ, Cronin A, et al.: Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med 367 (17): 1616-25, 2012.
-
Liu PH, Landrum MB, Weeks JC, et al.: Physicians' propensity to discuss prognosis is associated with patients' awareness of prognosis for metastatic cancers. J Palliat Med 17 (6): 673-82, 2014.
-
Craft PS, Burns CM, Smith WT, et al.: Knowledge of treatment intent among patients with advanced cancer: a longitudinal study. Eur J Cancer Care (Engl) 14 (5): 417-25, 2005.
-
Emanuel EJ, Emanuel LL: Four models of the physician-patient relationship. JAMA 267 (16): 2221-6, 1992 Apr 22-29.
-
McKneally MF, Martin DK: An entrustment model of consent for surgical treatment of life-threatening illness: perspective of patients requiring esophagectomy. J Thorac Cardiovasc Surg 120 (2): 264-9, 2000.
-
Schildmann J, Ritter P, Salloch S, et al.: 'One also needs a bit of trust in the doctor ... ': a qualitative interview study with pancreatic cancer patients about their perceptions and views on information and treatment decision-making. Ann Oncol 24 (9): 2444-9, 2013.
-
Epstein RM, Peters E: Beyond information: exploring patients' preferences. JAMA 302 (2): 195-7, 2009.
-
Agren Bolmsjö I, Nilstun T, Löfmark R: From cure to palliation: agreement, timing, and decision making within the staff. Am J Hosp Palliat Care 24 (5): 366-70, 2007 Oct-Nov.
-
Gramling R, Norton SA, Ladwig S, et al.: Direct observation of prognosis communication in palliative care: a descriptive study. J Pain Symptom Manage 45 (2): 202-12, 2013.
-
Matsuyama R, Reddy S, Smith TJ: Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24 (21): 3490-6, 2006.
-
Pellegrino ED: Decisions to withdraw life-sustaining treatment: a moral algorithm. JAMA 283 (8): 1065-7, 2000.
-
Walling A, Lorenz KA, Dy SM, et al.: Evidence-based recommendations for information and care planning in cancer care. J Clin Oncol 26 (23): 3896-902, 2008.
-
Wenrich MD, Curtis JR, Shannon SE, et al.: Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med 161 (6): 868-74, 2001.
-
Finucane TE: How gravely ill becomes dying: a key to end-of-life care. JAMA 282 (17): 1670-2, 1999.
Changes to This Summary (03 / 01 / 2017)The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Overview Revised text to state that the American Cancer Society estimates that more than 600,000 Americans will die of cancer in 2017 (cited American Cancer Society 2017 as reference 1). Factors That Influence End-of-Life Care Decisions and Outcomes Added American Cancer Society 2017 as reference 7. This summary is written and maintained by the PDQ Supportive and Palliative Care Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages. About This PDQ SummaryPurpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about planning for end-of-life care in advanced cancer. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Supportive and Palliative Care Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: - be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Planning the Transition to End-of-Life Care in Advanced Cancer are: - Mary K. Buss, MD, MPH (Beth Israel Deconess Medical Center)
- Larry D. Cripe, MD (Indiana University School of Medicine)
- David Hui, MD, MSC (M.D. Anderson Cancer Center)
- Maria Petzel, RD, CSO, LD, CNSC, FAND (University of TX MD Anderson Cancer Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Supportive and Palliative Care Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Supportive and Palliative Care Editorial Board. PDQ Planning the Transition to End-of-Life Care in Advanced Cancer. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/about-cancer/advanced-cancer/planning/end-of-life-hp-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389513] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2017-03-01 Last modified on: 8 September 2017
|
|