Childhood Liver Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
Childhood Liver Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]Skip to the navigationGeneral Information About Childhood Liver CancerFortunately, cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life: - Primary care physicians.
- Pediatric surgeons.
- Radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.) Guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer have been outlined by the American Academy of Pediatrics.[2] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients/families. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website. Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because late effects of therapy may persist or develop months or years after treatment. (Refer to Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.) Liver cancer is a rare malignancy in children and adolescents and is divided into the following two major histologic subgroups: - Hepatoblastoma.
- Hepatocellular carcinoma.
Other, less common, histologies include the following: - Undifferentiated embryonal sarcoma of the liver.
- Infantile choriocarcinoma of the liver.
References:
-
Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
-
Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004.
Cellular Classification of Childhood Liver CancerLiver tumors are rare in children. Their diagnoses may be challenging, in part, because of the lack of consensus regarding a classification system. Systematic central histopathological review of these tumors performed as part of pediatric collaborative therapeutic protocols has allowed the identification of histologic subtypes with distinct clinical associations. As a result, histopathology has been incorporated within the Children's Oncology Group (COG) protocols and, in the United States, as a risk-stratification parameter used for patient management. The COG Liver Tumor Committee sponsored an International Pathology Symposium in 2011 to discuss the histopathology and classification of pediatric liver tumors (hepatoblastoma, in particular) and work towards an International Pediatric Liver Tumors Consensus Classification that would be required for international collaborative projects. Twenty-two pathologists and experts in pediatric liver tumors, including those serving as central reviewers for the COG, European Société Internationale d'Oncologie Pédiatrique (International Society of Paediatric Oncology), Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology), and Japanese Study Group for Pediatric Liver Tumors protocols, as well as pediatric oncologists and surgeons specialized in this field, reviewed more than 50 pediatric liver tumor cases. They discussed classic and newly reported entities, and criteria for their classification. This symposium represented the first collaborative step toward developing a classification that may lead to a common treatment-stratification system incorporating tumor histopathology. The results of this international classification for pediatric liver tumors have been published.[1] It is too soon to know whether the international classification system will be generally accepted among pediatric pathologists. A standardized, clinically meaningful classification is needed to allow the integration of new biological parameters and tumor genetics, which could improve future patient management and outcome. For information on the histology of each childhood liver cancer subtype, refer to the following sections of this summary: - Hepatoblastoma.
- Hepatocellular carcinoma.
- Undifferentiated embryonal sarcoma of the liver.
- Infantile choriocarcinoma of the liver.
Genomic Abnormalities in Hepatoblastoma and Hepatocellular Carcinoma Genomic abnormalities related to hepatoblastoma include the following: - Hepatoblastoma mutation frequency, as determined by three groups using whole-exome sequencing, was very low (approximately three variants per tumor) in children younger than 5 years.[2,3,4]
- Hepatoblastoma is primarily a disease of WNT pathway activation. The primary mechanism for WNT pathway activation is CTNNB1 activating mutations/deletions involving exon 3. CTNNB1 mutations have been reported in 70% of cases.[2] Rare causes of WNT pathway activation include mutations in AXIN1, AXIN2, and APC (APC seen only in cases associated with familial adenomatosis polyposis coli).[5]
- The frequency of NFE2L2 mutations in hepatoblastoma specimens was reported to be 4 (7%) of 62 tumors in one study [3] and 5 (10%) of 51 specimens in another study.[2] Similar mutations have been found in many types of cancer including hepatocellular carcinoma. These mutations render NFE2L2 insensitive to KEAP1-mediated degradation, leading to activation of the NFE2L2-KEAP1 pathway, which activates resistance to oxidative stress and is believed to confer resistance to chemotherapy.
- Somatic mutations were identified in other genes related to regulation of oxidative stress, including inactivating mutations in the thioredoxin-domain containing genes, TXNDC15 and TXNDC16.[3]
- Figure 1 shows the distribution of CTNNB1, NFE2L2, and TERT mutations for hepatoblastoma.[2]
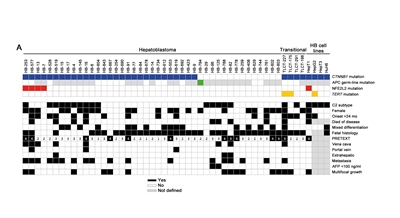
Figure 1. Mutational status and functional relevance of NFE2L2 in hepatoblastoma. Clinicopathological characteristics and the mutational status of the CTNNB1, APC, and NFE2L2 genes, as well as the TERT promoter region are color-coded and depicted in rows for each tumor of our cohort of 43 hepatoblastoma (HB) patients and four transitional liver cell tumour (TLCT) patients and 4 HB cell lines. Reprinted from Journal of Hepatology, Volume 61 (Issue 6), Melanie Eichenmüller, Franziska Trippel, Michaela Kreuder, Alexander Beck, Thomas Schwarzmayr, Beate Häberle, Stefano Cairo, Ivo Leuschner, Dietrich von Schweinitz, Tim M. Strom, Roland Kappler, The genomic landscape of hepatoblastoma and their progenies with HCC-like features, Pages 1312-1320, Copyright 2014, with permission from Elsevier.
Genomic abnormalities related to hepatocellular carcinoma include the following: - A first case of pediatric hepatocellular carcinoma was analyzed by whole-exome sequencing, which showed a higher mutation rate (53 variants) and the coexistence of CTNNB1 and NFE2L2 mutations.[6]
- Fibrolamellar hepatocellular carcinoma, a rare subtype of hepatocellular carcinoma observed in older children, is characterized by an approximately 400 kB deletion on chromosome 19 that results in production of a chimeric RNA coding for a protein containing the amino-terminal domain of DNAJB1, a homolog of the molecular chaperone DNAJ, fused in frame with PRKACA, the catalytic domain of protein kinase A.[7]
- A rare, more aggressive subtype of childhood liver cancer (hepatocellular carcinoma, not otherwise specified, also termed transitional liver cell tumor) occurs in older children, and it has clinical and histopathological findings of both hepatoblastoma and hepatocellular carcinoma. TERT mutations were observed in two of four cases tested.[2]TERT mutations are also commonly observed in adults with hepatocellular carcinoma.[8]
References:
-
López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
-
Eichenmüller M, Trippel F, Kreuder M, et al.: The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol 61 (6): 1312-20, 2014.
-
Trevino LR, Wheeler DA, Finegold MJ, et al.: Exome sequencing of hepatoblastoma reveals recurrent mutations in NFE2L2. [Abstract] Cancer Res 73 (8 Suppl): A-4592, 2013. Also available online. Last accessed February 03, 2017.
-
Jia D, Dong R, Jing Y, et al.: Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 60 (5): 1686-96, 2014.
-
Hiyama E, Kurihara S, Onitake Y: Integrated exome analysis in childhood hepatoblastoma: Biological approach for next clinical trial designs. [Abstract] Cancer Res 74 (19 Suppl): A-5188, 2014.
-
Vilarinho S, Erson-Omay EZ, Harmanci AS, et al.: Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. J Hepatol 61 (5): 1178-83, 2014.
-
Honeyman JN, Simon EP, Robine N, et al.: Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343 (6174): 1010-4, 2014.
-
Nault JC, Mallet M, Pilati C, et al.: High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 4: 2218, 2013.
Tumor Stratification by Imaging and Postsurgical Staging for Childhood Liver CancerHistorically, the four major study groups (International Childhood Liver Tumors Strategy Group [previously known as Société Internationale d'Oncologie Pédiatrique-Epithelial Liver Tumor Study Group (SIOPEL)], Children's Oncology Group [COG], Gesellschaft für Pädiatrische Onkologie und Hämatologie [Society for Paediatric Oncology and Haematology], and Japanese Study Group for Pediatric Liver Tumors) have had disparate risk stratification categories, making it difficult to compare outcomes across continents. All groups are now using the PRE-Treatment EXTent of tumor (PRETEXT) grouping system as part of the risk stratification. The primary treatment goal for patients with liver cancer is surgical extirpation of all disease. Therefore, the risk grouping designed to stratify treatment depends heavily on factors related to safe surgical resection of the tumor. This risk grouping uses imaging to define factors that determine the likelihood of safe and successful surgical resection. The importance of high-quality, cross-sectional imaging to evaluate children with hepatoblastoma is paramount because the risk stratification that defines treatment is very dependent on imaging analysis. Three-phase computed tomography scanning (non-contrast, arterial, and venous) or magnetic resonance imaging (MRI) with contrast agents are used for imaging. MRI with gadoxetate disodium (Eovist), a gadolinium-based agent that is preferentially taken up and excreted by hepatocytes, is being used with increased frequency and may improve detection of multifocal disease. There are two grouping systems used for hepatoblastoma and hepatocellular carcinoma that radiographically define the extent of liver involvement by the tumor: - PRETEXT (PRE-Treatment EXTent of disease): The extent of liver involvement is defined before therapy.
- POSTTEXT (POST-Treatment EXTent of disease): The extent of liver involvement is defined after therapy.
In SIOPEL studies, all children with hepatoblastoma have been treated with chemotherapy before attempted resection of the primary tumor. Hence, surgical staging has not been possible. PRETEXT and POSTTEXT Groups PRETEXT is now used by the major multicenter trial groups as a central component of risk stratification schemes that define treatment of hepatoblastoma. The PRETEXT groups were devised by the SIOPEL for their first trial, SIOPEL-1 [1] and revised for SIOPEL-3 in 2007.[2] PRETEXT is based on an analysis of cross-sectional imaging of the extent of tumor involvement of the four main sections of the liver: - Right posterior section (Couinaud 6, 7).
- Right anterior section (Couinaud 5, 8).
- Left medial section (Couinaud 4a, 4b).
- Left lateral section (Couinaud 2, 3).
PRETEXT group assignment I, II, III, or IV is determined by the number of contiguous uninvolved sections of the liver. PRETEXT is further annotated with a V, P, E, M, C, F, N, or R depending on extension of tumor beyond the hepatic parenchyma of the major sections. Annotations have been added to identify multifocality (F) and preoperative tumor rupture (R). (Refer to Table 1 for detailed descriptions of the PRETEXT groups and annotations.) The extent of tumor involvement of the major vessels and its effect on venous inflow and outflow is critical knowledge for the surgeon and can affect surgical outcomes. Vascular involvement is critical in determining the resectability of a liver tumor. It should be noted that there are differences in the definitions of vascular involvement used by the COG and major liver surgery centers in the United States compared with SIOPEL definitions used in Europe. Although PRETEXT can be used to predict tumor resectability, there are limitations. The distinction between real invasion beyond the anatomic border of a given hepatic section and the compression and displacement by the tumor can be very difficult, especially at diagnosis. Additionally, distinguishing between vessel encroachment and involvement can be difficult, particularly if inadequate imaging is obtained. The PRETEXT group assignment has a moderate degree of interobserver variability, and the preoperative PRETEXT group agrees with postoperative pathologic findings only 51% of the time, with overstaging in 37% of patients and understaging in 12% of patients.[3] Because distinguishing PRETEXT group assignment is difficult, central review of imaging is generally performed in major clinical trials. For patients not enrolled on clinical trials, expert radiologic review should be considered in questionable cases in which the PRETEXT group assignment affects choice of treatment. The posttreatment extent of disease (POSTTEXT) is typically obtained after every two cycles of chemotherapy, about 10 days after the completion of a chemotherapy cycle. It has been shown that most chemotherapy response occurs after the first two cycles of chemotherapy.[4,5] Also, a study that evaluated surgical resectability after two versus four cycles of chemotherapy showed that many tumors may be resectable after two cycles.[4] Table 1. Definitions of PRETEXT and POSTTEXT Groups and Annotationsa| PRETEXT and POSTTEXT Groups | Definition | Image |
|---|
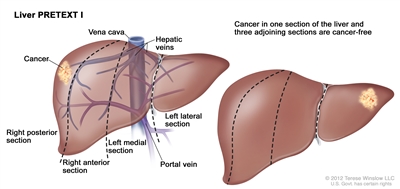
| a Adapted from Roebuck et al.[2] | | I | One section involved; three adjoining sections are tumor free. |
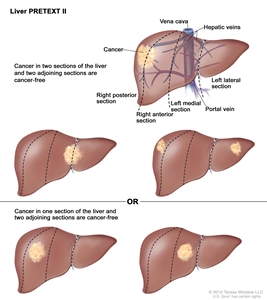
| | II | One or two sections involved; two adjoining sections are tumor free. |
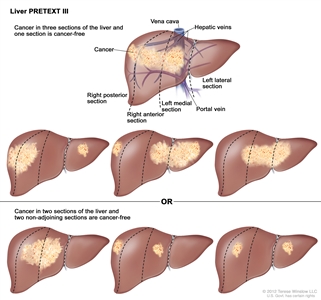
| | III | Two or three sections involved; one adjoining section is tumor free. |
| | IV | Four sections involved. |
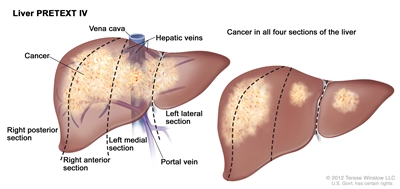
| | Annotation | | | V | Venous involvement: Vascular involvement of the retrohepatic vena cava or involvement ofall three major hepatic veins (right, middle, and left). | | | V0 | -Tumor within 1 cm. | | | V1 | -Tumor touching. | | | V2 | -Tumor compressing or distorting. | | | V3 | -Tumor ingrowth, encasement, or thrombus. | | P | Portal involvement: Vascular involvement of the main portal vein and/orboth right and left portal veins. | | | P0 | -Tumor within 1 cm. | | | P1 | -Tumor touching. | | | P2 | -Tumor compressing or distorting. | | | P3 | -Tumor ingrowth, encasement, or thrombus. | | E | Extrahepatic involvement of a contiguous structure such as the diaphragm, abdominal wall, stomach, colon, etc. | | | E1 | -Direct extension of tumor in adjacent organs or diaphragm. | | | E2 | -Peritoneal nodules (add a suffix to E if any tumor ascites). | | M | Distant metastatic disease (usually lungs, occasionally bone or brain). | | C | Caudate lobe involvement. | | | C1 | -Tumor involving the caudate lobe (all C1 patients are at least PRETEXT II). | | F | Multifocal tumor nodules. | | | F1 | -Two or more discrete tumors (multifocal). | | N | Lymph node involvement. | | | N1 | -Abdominal lymph node metastasis only. | | | N2 | -Extra-abdominal lymph node metastasis (with or without abdominal nodes). | | R | Tumor rupture. | | H1 | Imaging and clinical findings of intraperitoneal hemorrhage. | | M1 | Any metastasis other than E or N. | Hepatoblastoma and hepatocellular carcinoma prognosis by PRETEXT group The 5-year overall survival (OS) in the first international study of hepatoblastoma, in which the study protocol called for treatment of children with preoperative doxorubicin and cisplatin chemotherapy and included children with metastasis, was as follows:[6,7] - 100% for PRETEXT I.
- 91% for PRETEXT II.
- 68% for PRETEXT III.
- 57% for PRETEXT IV.
- 25% for patients with metastasis.
The second international study compared 3-year OS among hepatoblastoma patients without extrahepatic disease by PRETEXT group. The 3-year OS was as follows:[8] - 100% for PRETEXT I.
- 95% for PRETEXT II.
- 84% for PRETEXT III.
- 61% for PRETEXT IV.
The study also prospectively analyzed patients' OS by the presence of intraabdominal extrahepatic disease without distant metastasis (OS, 58%) and with distant metastases (OS, 44%).[8] Patients who underwent orthotopic liver transplant are included in all of the international study results.[9] The 5-year OS by PRETEXT group for hepatocellular carcinoma was as follows:[10] - 44% for PRETEXT I.
- 44% for PRETEXT II.
- 22% for PRETEXT III.
- 8% for PRETEXT IV.
The COG is investigating prospective grouping of hepatoblastoma patients using the PRETEXT system to determine the timing of surgery and the timing of early notification of liver transplant centers (COG-AHEP0731). Postsurgical Staging for Childhood Liver Cancer (Historical) A staging system based on operative findings and surgical resectability was used for many years in the United States to group children with liver cancer. This staging system was used to determine treatment.[11,12,13] Currently other risk stratification systems are used to classify patients and determine treatment strategy (refer to Table 3 for more information). Hepatoblastoma prognosis by postsurgical stage Stages I and II In stage I hepatoblastoma, the tumor is completely resected. In stage II hepatoblastoma, microscopic residual tumor remains after resection. Approximately 20% to 30% of children with hepatoblastoma are stage I or II. Prognosis varies depending on the subtype of hepatoblastoma: - Pure fetal histology (4% of hepatoblastomas) have a 3- to 5-year OS rate of 100% with minimal or no chemotherapy.[13,14,15]
- Non-pure fetal histology, non-small cell undifferentiated stage I and II hepatoblastomas have a 3- to 4-year OS rate of 90% to 100% with adjuvant chemotherapy.[6,8,13,14,16]
- If any small cell undifferentiated elements are present in stage I or II hepatoblastoma, the 3-year survival rate is 40% to 70%.[14,17]
Stage III In stage III hepatoblastoma, there are no distant metastases and one of the following is true: - The tumor is either unresectable or the tumor is resected with gross residual tumor.
- There are positive lymph nodes.
Approximately 50% to 70% of children with hepatoblastoma are stage III. The 3- to 5-year OS rate for children with stage III hepatoblastoma is less than 70%.[6,8,13,14,18] Stage IV (distant metastases) In stage IV hepatoblastoma, there is distant metastasis regardless of the extent of liver involvement. Approximately 10% to 20% of children with hepatoblastoma are stage IV. The 3- to 5-year OS rate for children with stage IV hepatoblastoma varies widely, from 20% to approximately 60%, based on published reports.[6,7,8,13,14,18] Hepatocellular carcinoma prognosis by postsurgical stage - Children with stage I hepatocellular carcinoma have a good outcome.[19]
- Stage II is too rarely seen to predict outcome.
- Stages III and IV are usually fatal.[10,20]
References:
-
Brown J, Perilongo G, Shafford E, et al.: Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36 (11): 1418-25, 2000.
-
Roebuck DJ, Aronson D, Clapuyt P, et al.: 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 37 (2): 123-32; quiz 249-50, 2007.
-
Aronson DC, Schnater JM, Staalman CR, et al.: Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 23 (6): 1245-52, 2005.
-
Lovvorn HN 3rd, Ayers D, Zhao Z, et al.: Defining hepatoblastoma responsiveness to induction therapy as measured by tumor volume and serum alpha-fetoprotein kinetics. J Pediatr Surg 45 (1): 121-8; discussion 129, 2010.
-
Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
-
Pritchard J, Brown J, Shafford E, et al.: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18 (22): 3819-28, 2000.
-
Perilongo G, Brown J, Shafford E, et al.: Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 89 (8): 1845-53, 2000.
-
Perilongo G, Shafford E, Maibach R, et al.: Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 40 (3): 411-21, 2004.
-
Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
-
Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
-
Ortega JA, Krailo MD, Haas JE, et al.: Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (12): 2167-76, 1991.
-
Douglass EC, Reynolds M, Finegold M, et al.: Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol 11 (1): 96-9, 1993.
-
Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000.
-
Meyers RL, Rowland JR, Krailo M, et al.: Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 53 (6): 1016-22, 2009.
-
Malogolowkin MH, Katzenstein HM, Meyers RL, et al.: Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol 29 (24): 3301-6, 2011.
-
Perilongo G, Maibach R, Shafford E, et al.: Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 361 (17): 1662-70, 2009.
-
De Ioris M, Brugieres L, Zimmermann A, et al.: Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer 44 (4): 545-50, 2008.
-
Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010.
-
Douglass E, Ortega J, Feusner J, et al.: Hepatocellular carcinoma (HCA) in children and adolescents: results from the Pediatric Intergroup Hepatoma Study (CCG 8881/POG 8945). [Abstract] Proceedings of the American Society of Clinical Oncology 13: A-1439, 420, 1994.
-
Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 20 (12): 2789-97, 2002.
Treatment Option Overview for Childhood Liver CancerMany of the improvements in survival in childhood cancer have been made using new therapies that have attempted to improve on the best available, accepted therapy. Clinical trials in pediatrics are designed to compare potentially better therapy with therapy that is currently accepted as standard. This comparison may be done in a randomized study of two treatment arms or by evaluating a single new treatment, comparing the results with those previously obtained with standard therapy. Because of the relative rarity of cancer in children, all children with liver cancer should be considered for entry onto a clinical trial. Treatment planning by a multidisciplinary team of cancer specialists with experience treating tumors of childhood is required to determine and implement optimal treatment.[1] Surgery Historically, complete surgical resection of the primary tumor has been required to cure malignant liver tumors in children.[2,3,4,5,6]; [7][Level of evidence: 3iiiA] This approach continues to be the goal of definitive surgical procedures, and surgical resection is often combined with other treatment modalities (e.g., liver transplant, chemotherapy). However, postoperative complications are common and associated with worsened overall survival in patients with advanced hepatoblastoma.[8] There are three ways in which surgery is used to treat primary pediatric liver cancer: - Initial surgical resection (alone or followed by chemotherapy).
- Delayed surgical resection (preceded by chemotherapy).
- Orthotopic liver transplantation.
The timing of the surgical approach is critical. For this reason, surgeons with experience in pediatric liver resection and transplantation are involved early in the decision-making process for determining optimal timing and extent of resection. In children and adolescents with primary liver tumors, the surgeon has to be prepared to perform a highly sophisticated liver resection after confirmation of the diagnosis by pathological investigation of intraoperative frozen sections. While complete surgical resection is important for all liver tumors, it is especially true for hepatocellular carcinoma because curative chemotherapy is not available. Intraoperative ultrasound may result in further delineation of tumor extent and location and can affect intraoperative management.[9] If the tumor can be completely excised by an experienced surgical team, less postoperative chemotherapy may be needed. If the tumor is determined to be unresectable and preoperative chemotherapy is to be administered, it is very important to frequently consult with the surgical team concerning the timing of resection, as prolonged chemotherapy can lead to unnecessary delays and, in rare cases, tumor progression. Early involvement with an experienced pediatric liver surgeon is especially important in patients with PRETEXT III or IV disease, involvement of major liver vessels (V+ [venous] or P+ [portal]), or low alpha-fetoprotein (AFP) levels.[10] Although vascular involvement was initially thought to be a contraindication to resection, experienced liver surgeons are often able to perform aggressive approaches while avoiding transplantation.[10,11]; [12][Level of evidence: 3iiA] Accomplishing a complete resection is imperative because rescue transplant of incompletely resected patients has an inferior outcome compared with patients who are transplanted as the primary surgical therapy.[13] The decision as to which surgical approach to use depends on many factors including the following: - PRETEXT group and POSTTEXT group.
- Size of the primary tumor.
- Presence of multifocal hepatic disease.
- Vascular involvement.
- AFP levels.
- Whether preoperative chemotherapy is likely to convert an unresectable tumor into a potentially resectable tumor.
- Whether hepatic disease meets surgical and histopathologic criteria for orthotopic liver transplantation.
The approach taken by the Children's Oncology Group (COG) in North American clinical trials is to perform surgery initially when a complete resection can be accomplished with a simple, negative-margin hemihepatectomy. The COG has studied the use of PRETEXT and POSTTEXT to determine the optimal approach and timing of surgery. POSTTEXT imaging grouping is performed after two and four cycles of chemotherapy to determine the optimal time for definitive surgery (refer to the Tumor Stratification by Imaging and Postsurgical Staging for Childhood Liver Cancer section of this summary for more information).[4,14] Orthotopic liver transplantation Liver transplantation has recently been associated with significant success in the treatment of children with unresectable hepatic tumors.[15,16,17][Level of evidence: 3iiA] A review of the world experience has documented a posttransplant survival rate of 70% to 80% for children with hepatoblastomas.[13,18,19] Intravenous invasion, positive lymph nodes, and contiguous spread did not have a significant adverse effect on outcome. It has been suggested that adjuvant chemotherapy after transplant may decrease the risk of tumor recurrence.[20] Evidence (orthotopic transplantation): - The United Network for Organ Sharing (UNOS) Standard Transplant and Research Files registry reported all children younger than 18 years listed for a liver transplant in the United States from October 1987 through July 2004. Of these children, 135 had hepatoblastoma and 41 had hepatocellular carcinoma and both groups received liver transplant.[21,22]
- Five-year survival rates of 69% were reported for hepatoblastoma and 63% for hepatocellular carcinoma.
- The 10-year survival rates were similar to the 5-year rates.
- In a three-institution study of children with hepatocellular carcinoma, the overall 5-year disease-free survival rate was approximately 60%.[23]
Application of the Milan criteria for UNOS selection of recipients of deceased donor livers is controversial.[24] The Milan criteria for liver transplantation are directed toward adults with cirrhosis and hepatocellular carcinoma. The criteria do not apply to children and adolescents with hepatocellular carcinoma, especially those without cirrhosis. Living-donor liver transplant is more common with children and the outcome is similar to those receiving cadaveric liver transplant.[25,26] In hepatocellular carcinoma, vascular invasion, distant metastases, lymph node involvement, tumor size, and male gender were significant risk factors for recurrence. Because of the poor prognosis in patients with hepatocellular carcinoma, liver transplant should be considered for disorders such as tyrosinemia and familial intrahepatic cholestasis early in the course, before the development of liver failure and malignancy. Surgical resection for metastatic disease Surgical resection of distant disease has also contributed to the cure of children with hepatoblastoma. Resection of pulmonary metastases is recommended when the number of metastases is limited [27,28,29] and is often performed at the same time as resection of the primary tumor. When possible, resection of areas of locally invasive disease, such as in the diaphragm, and of isolated brain metastasis is recommended.[30] Chemotherapy Chemotherapy regimens used in the treatment of hepatoblastoma and hepatocellular carcinoma are described in their respective sections (refer to the Treatment of Hepatoblastoma and the Treatment of Hepatocellular Carcinoma sections of this summary for more information). Chemotherapy has been much more successful in the treatment of hepatoblastoma than in hepatocellular carcinoma.[4,5,31,32,33,34,35] The standard of care in the United States is preoperative chemotherapy when the tumor is unresectable and postoperative chemotherapy after complete resection, even if preoperative chemotherapy has already been given. Preoperative chemotherapy has been shown to be of benefit in children with hepatoblastoma; however, the use of postoperative chemotherapy after definitive surgical resection or liver transplant has not been investigated in a randomized fashion. Radiation Therapy The utility of radiation therapy is questioned because the liver cannot tolerate high doses of radiation.[32,36] Radiation therapy, even in combination with chemotherapy, has not cured children with unresectable tumors. Radiation therapy may have a role in the management of incompletely resected hepatoblastoma,[32,36] although a study of 154 patients with hepatoblastoma did not confirm this finding.[37] This study showed that radiation therapy and/or second resection of positive margins may not be necessary in patients with incompletely resected hepatoblastoma whose residual tumor is microscopic.[37] Other Treatment Approaches Other treatment approaches such as transarterial chemoembolization (TACE) have been used for patients with inoperable hepatoblastoma.[38,39] TACE has been used in a few children to successfully shrink the tumor to permit resection.[39] Chemotherapy followed by TACE followed by high-intensity focused ultrasound showed promising results in China for PRETEXT III and IV patients, some of whom were resectable but did not undergo surgery because of parent refusal.[40] Transarterial radioembolization with yttrium-90 resin beads has been used to palliate children with hepatocellular carcinoma.[41] (Refer to the PDQ summary on Adult Primary Liver Cancer Treatment for more information.) References:
-
Tiao GM, Bobey N, Allen S, et al.: The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr 146 (2): 204-11, 2005.
-
Czauderna P, Otte JB, Aronson DC, et al.: Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer 41 (7): 1031-6, 2005.
-
Exelby PR, Filler RM, Grosfeld JL: Liver tumors in children in the particular reference to hepatoblastoma and hepatocellular carcinoma: American Academy of Pediatrics Surgical Section Survey--1974. J Pediatr Surg 10 (3): 329-37, 1975.
-
Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 20 (12): 2789-97, 2002.
-
Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
-
Meyers RL, Czauderna P, Otte JB: Surgical treatment of hepatoblastoma. Pediatr Blood Cancer 59 (5): 800-8, 2012.
-
Allan BJ, Wang B, Davis JS, et al.: A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 49 (1): 166-71; discussion 171, 2014.
-
Becker K, Furch C, Schmid I, et al.: Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr Blood Cancer 62 (1): 24-8, 2015.
-
Felsted AE, Shi Y, Masand PM, et al.: Intraoperative ultrasound for liver tumor resection in children. J Surg Res 198 (2): 418-23, 2015.
-
D'Antiga L, Vallortigara F, Cillo U, et al.: Features predicting unresectability in hepatoblastoma. Cancer 110 (5): 1050-8, 2007.
-
Hemming AW, Reed AI, Fujita S, et al.: Role for extending hepatic resection using an aggressive approach to liver surgery. J Am Coll Surg 206 (5): 870-5; discussion 875-8, 2008.
-
Baertschiger RM, Ozsahin H, Rougemont AL, et al.: Cure of multifocal panhepatic hepatoblastoma: is liver transplantation always necessary? J Pediatr Surg 45 (5): 1030-6, 2010.
-
Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
-
Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
-
Guiteau JJ, Cotton RT, Karpen SJ, et al.: Pediatric liver transplantation for primary malignant liver tumors with a focus on hepatic epithelioid hemangioendothelioma: the UNOS experience. Pediatr Transplant 14 (3): 326-31, 2010.
-
Malek MM, Shah SR, Atri P, et al.: Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery 148 (4): 778-82; discussion 782-4, 2010.
-
Héry G, Franchi-Abella S, Habes D, et al.: Initial liver transplantation for unresectable hepatoblastoma after chemotherapy. Pediatr Blood Cancer 57 (7): 1270-5, 2011.
-
Suh MY, Wang K, Gutweiler JR, et al.: Safety of minimal immunosuppression in liver transplantation for hepatoblastoma. J Pediatr Surg 43 (6): 1148-52, 2008.
-
Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010.
-
Browne M, Sher D, Grant D, et al.: Survival after liver transplantation for hepatoblastoma: a 2-center experience. J Pediatr Surg 43 (11): 1973-81, 2008.
-
Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006.
-
Heaton N, Faraj W, Melendez HV, et al.: Living related liver transplantation in children. Br J Surg 95 (7): 919-24, 2008.
-
Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
-
Otte JB: Should the selection of children with hepatocellular carcinoma be based on Milan criteria? Pediatr Transplant 12 (1): 1-3, 2008.
-
Sevmis S, Karakayali H, Ozçay F, et al.: Liver transplantation for hepatocellular carcinoma in children. Pediatr Transplant 12 (1): 52-6, 2008.
-
Faraj W, Dar F, Marangoni G, et al.: Liver transplantation for hepatoblastoma. Liver Transpl 14 (11): 1614-9, 2008.
-
Feusner JH, Krailo MD, Haas JE, et al.: Treatment of pulmonary metastases of initial stage I hepatoblastoma in childhood. Report from the Childrens Cancer Group. Cancer 71 (3): 859-64, 1993.
-
Zsiros J, Brugieres L, Brock P, et al.: Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14 (9): 834-42, 2013.
-
Meyers RL, Katzenstein HM, Krailo M, et al.: Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42 (12): 2050-6, 2007.
-
Robertson PL, Muraszko KM, Axtell RA: Hepatoblastoma metastatic to brain: prolonged survival after multiple surgical resections of a solitary brain lesion. J Pediatr Hematol Oncol 19 (2): 168-71, 1997 Mar-Apr.
-
Ortega JA, Krailo MD, Haas JE, et al.: Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (12): 2167-76, 1991.
-
Douglass EC, Reynolds M, Finegold M, et al.: Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol 11 (1): 96-9, 1993.
-
Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000.
-
Pritchard J, Brown J, Shafford E, et al.: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18 (22): 3819-28, 2000.
-
Perilongo G, Shafford E, Maibach R, et al.: Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 40 (3): 411-21, 2004.
-
Habrand JL, Nehme D, Kalifa C, et al.: Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23 (3): 525-31, 1992.
-
Schnater JM, Aronson DC, Plaschkes J, et al.: Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 94 (4): 1111-20, 2002.
-
Xianliang H, Jianhong L, Xuewu J, et al.: Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol 26 (1): 60-3, 2004.
-
Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
-
Wang S, Yang C, Zhang J, et al.: First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 59 (1): 170-7, 2014.
-
Hawkins CM, Kukreja K, Geller JI, et al.: Radioembolisation for treatment of pediatric hepatocellular carcinoma. Pediatr Radiol 43 (7): 876-81, 2013.
HepatoblastomaIncidence The annual incidence of hepatoblastoma in the United States appears to have doubled from 0.8 (1975-1983) to 1.6 (2002-2009) per 1 million children aged 19 years and younger.[1,2] The cause for this increase is unknown, but the increasing survival of very low-birth-weight premature infants, which is known to be associated with hepatoblastoma, may contribute.[3] In Japan, the risk of hepatoblastoma in children who weighed less than 1,000 g at birth is 15 times the risk in normal birth-weight children.[4] Other data has confirmed the high incidence of hepatoblastoma in very low-birth-weight premature infants.[5] Attempts to identify factors resulting from treatment of infants born prematurely have not revealed any suggestive causation of the increased incidence of hepatoblastoma.[3] The age of onset of liver cancer in children is related to tumor histology. Hepatoblastomas usually occur before the age of 3 years, and approximately 90% of malignant liver tumors in children aged 4 years and younger are hepatoblastomas.[6] Risk Factors Conditions associated with an increased risk of hepatoblastoma are described in Table 2. Table 2. Conditions Associated With Hepatoblastoma| Associated Disorder | Clinical Findings |
|---|
| Aicardi syndrome[7] | Refer to the Aicardi syndromesection of this summary for more information. | | Beckwith-Wiedemann syndrome[8,9] | Refer to the Beckwith-Wiedemann syndrome and hemihyperplasiasection of this summary for more information. | | Familial adenomatous polyposis[10,11,12] | Refer to the Familial adenomatous polyposissection of this summary for more information. | | Glycogen storage diseases I-IV[13] | Symptoms vary by individual disorder. | | Low-birth-weight infants[3,4,5,14,15] | Preterm and small-for-gestation-age neonates. | | Simpson-Golabi-Behmel syndrome[16] | Macroglossia, macrosomia, renal and skeletal abnormalities, and increased risk of Wilms tumor. | | Trisomy 18, other trisomies[17] | Trisomy 18: Microcephaly and micrognathia, clenched fists with overlapping fingers, and failure to thrive. Most patients (>90%) die in the first month of life. | Aicardi syndrome Aicardi syndrome is presumed to be an X-linked condition reported exclusively in females, leading to the hypothesis that a mutated gene on the X chromosome causes lethality in males. The syndrome is classically defined as agenesis of the corpus callosum, chorioretinal lacunae, and infantile spasms, with a characteristic facies. Additional brain, eye, and costovertebral defects are often found.[7] Beckwith-Wiedemann syndrome and hemihyperplasia The incidence of hepatoblastoma is increased 1,000-fold to 10,000-fold in infants and children with Beckwith-Wiedemann syndrome.[9,18] Hepatoblastoma is also increased in hemihypertrophy, now termed hemihyperplasia, a condition that results in asymmetry between the right and left side of the body when a body part grows faster than normal.[19,20] Beckwith-Wiedemann syndrome is most commonly caused by epigenetic changes and is sporadic. The syndrome may also be caused by genetic mutations and be familial. Either mechanism can be associated with an increased incidence of embryonal tumors, including Wilms tumor and hepatoblastoma.[9] The expression of both IGFR2 alleles and ensuing increased expression of insulin-like growth factor 2 (IGF-2) has been implicated in the macrosomia and embryonal tumors in Beckwith-Wiedemann syndrome.[9,21] When sporadic, the types of embryonal tumors associated with Beckwith-Wiedemann syndrome have frequently also undergone somatic changes in the Beckwith-Wiedemann syndrome locus and IGF-2.[22,23] The genetics of tumors in children with hemihyperplasia have not been clearly defined. To detect abdominal malignancies at an early stage, all children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia are screened regularly for multiple tumor types by abdominal ultrasound.[20] Screening using alpha-fetoprotein (AFP) levels has also helped in the early detection of hepatoblastoma in these children.[24] Because hepatoblastoma in Beckwith-Wiedemann syndrome is detected at an early stage and tumors are small, it has been suggested to minimize treatment after surgery.[18] Familial adenomatous polyposis There is an association between hepatoblastoma and familial adenomatous polyposis (FAP); children in families that carry the APC gene have an 800-fold increased risk for hepatoblastoma. However, hepatoblastoma has been reported to occur in less than 1% of FAP family members, so screening for hepatoblastoma in members of families with FAP using ultrasound and AFP levels is controversial.[10,11,12,25] However, one study of 50 consecutive children with apparent sporadic hepatoblastoma reported five children (10%) had APC germline mutations.[25] Current evidence cannot rule out the possibility that predisposition to hepatoblastoma may be limited to a specific subset of APC mutations. Another study of children with hepatoblastoma found a predominance of the mutation in the 5' region of the gene, but some patients had mutations closer to the 3' region.[26] This preliminary study provides some evidence that screening children with hepatoblastoma for APC mutations and colon cancer may be appropriate. In the absence of APC germline mutations, childhood hepatoblastomas do not have somatic mutations in the APC gene; however, the hepatoblastomas frequently have mutations in the beta-catenin gene, the function of which is closely related to APC.[27] Diagnosis A biopsy of the tumor is always indicated to secure the diagnosis of a liver tumor except in the following circumstances: - In infantile hemangioendothelioma of the liver, which can be diagnosed by imaging.
- In infantile hepatic choriocarcinoma, which can be diagnosed by imaging and markedly elevated beta-human chorionic gonadotropin (beta-hCG).[28]
The AFP and beta-hCG tumor markers are very helpful in diagnosis and management of liver tumors. Although AFP is elevated in most children with hepatic malignancy, it is not pathognomonic for a malignant liver tumor.[29] The AFP level can be elevated with either a benign tumor or a malignant solid tumor. AFP is very high in neonates and steadily falls after birth. The half-life of AFP is 5 to 7 days, and by age 1 year, it should be less than 10 ng/mL.[30] Prognosis and Prognostic Factors The 5-year overall survival (OS) rate for children with hepatoblastoma is 70%.[31,32] Neonates with hepatoblastoma have comparable outcomes to older children up to age 5 years.[33] Individual childhood cancer study groups have attempted to define the relative importance of a variety of prognostic factors present at diagnosis and in response to therapy.[34,35] A collaborative group consisting of four study groups (International Childhood Liver Tumors Strategy Group [SIOPEL], Children's Oncology Group [COG], Gesellschaft für Pädiatrische Onkologie und Hämatologie [GPOH], and Japanese Study Group for Pediatric Liver Tumor [JPLT]), termed Childhood Hepatic tumor International Collaboration (CHIC), have retrospectively combined data from eight clinical trials (N = 1,605) conducted between 1988 and 2010. The CHIC published a univariate analysis of the effect of clinical prognostic factors present at the time of diagnosis on event-free survival (EFS).[36] The analysis confirmed many of the findings described below. The statistically significant adverse factors included the following:[36] - Higher PRE-Treatment EXTent of disease (PRETEXT) score.
- Multifocal primary.
- Macrovascular involvement.
- Portal vein involvement.
- Extrahepatic tumor, tumor rupture, and metastasis.
- Low AFP level (<100 ng/ml).
- Older age. Patients aged 8 years and older have a worse outcome than patients aged 3 to 7 years. Patients aged 3 to 7 years have a worse outcome than patients younger than 2 years.
In contrast, sex, prematurity, birth weight, and Beckwith-Wiedemann syndrome had no effect on EFS.[36] A multivariate analysis of these prognostic factors has been published to help develop a new risk group classification for hepatoblastoma.[37] This classification was used to generate a risk stratification schema to be used in international clinical trials. Other studies of factors affecting prognosis observed the following: - PRETEXT group: In SIOPEL studies, having a low PRETEXT group at diagnosis (PRETEXT I, II, and III tumors) is a good prognostic factor, whereas PRETEXT IV is a poor prognostic factor.[36] (Refer to the Tumor Stratification by Imaging and Postsurgical Staging for Childhood Liver Cancer section of this summary for more information.)
- Tumor stage: In COG studies, stage I tumors that were resected at diagnosis and tumors with pure fetal histology have a good prognosis. These tumors are treated differently than tumors of other stages and histologies.[36]
- Treatment-related factors:
Surgery: Cure of hepatoblastoma requires gross tumor resection. Hepatoblastoma is most often unifocal and thus, resection may be possible. If a hepatoblastoma is completely removed, the majority of patients survive, but because of vascular or other involvement, less than one-third of patients have lesions amenable to complete resection at diagnosis.[36] Thus, it is critically important that a child with probable hepatoblastoma be evaluated by a pediatric surgeon who is experienced in the resection of hepatoblastoma in children and has access to a liver transplant program. In advanced tumors, surgical treatment of hepatoblastoma is a demanding procedure. Postoperative complications in high-risk patients decrease the rate of overall survival.[38] Chemotherapy: Chemotherapy often decreases the size and extent of hepatoblastoma, allowing complete resection.[39,40,41,42,43] Orthotopic liver transplantation provides an additional treatment option for patients whose tumor remains unresectable after preoperative chemotherapy;[44,45] however, the presence of microscopic residual tumor at the surgical margin does not preclude a favorable outcome.[46,47] This may be due to the additional courses of chemotherapy that are administered before or after resection.[39,40,46] (Refer to Table 4 for more information on outcomes associated with specific chemotherapy regimens.) - Tumor marker-related factors:
Ninety percent of patients with hepatoblastoma and two-thirds of patients with hepatocellular carcinoma exhibit the serum tumor marker AFP, which parallels disease activity. The level of AFP at diagnosis and rate of decrease in AFP levels during treatment are compared with the age-adjusted normal range. Lack of a significant decrease of AFP levels with treatment may predict a poor response to therapy.[48] Absence of elevated AFP levels at diagnosis (AFP less than 100 ng/mL) occurs in a small percentage of children with hepatoblastoma and appears to be associated with very poor prognosis, as well as with the small cell undifferentiated variant of hepatoblastoma.[36] Some of these variants do not express INI1 due to INI1 mutation and may be considered rhabdoid tumors of the liver; all small cell undifferentiated hepatoblastomas are tested for loss of INI1 expression by immunohistochemistry.[49,50,51,52,53,54] Beta-hCG levels may also be elevated in children with hepatoblastoma or hepatocellular carcinoma, which may result in isosexual precocity in boys.[55,56] - Tumor histology:
Refer to the Histology section of this summary for more information.
Other variables have been suggested as poor prognostic factors, but the relative importance of their prognostic significance has been difficult to define. In the SIOPEL-1 study, a multivariate analysis of prognosis after positive response to chemotherapy showed only one variable, PRETEXT, predicted OS, while metastasis and PRETEXT predicted EFS.[49] In an analysis of the intergroup U.S. study from the time of diagnosis, pure fetal histology, small cell undifferentiated histology, and AFP less than 100 ng/mL were prognostic in a log rank analysis. PRETEXT was prognostic among patients designated group III, but not group IV.[53,57] Histology Hepatoblastoma arises from precursors of hepatocytes and can have several morphologies, including the following:[58] - Small cells that reflect neither epithelial nor stromal differentiation.
- Embryonal epithelial cells resembling the liver epithelium at 6 to 8 weeks of gestation.
- Well-differentiated fetal hepatocytes morphologically indistinguishable from normal fetal liver cells.
Most often the tumor consists of a mixture of epithelial hepatocyte precursors. About 20% of tumors have stromal derivatives such as osteoid, chondroid, and rhabdoid elements. Occasionally, neuronal, melanocytic, squamous, and enteroendocrine elements are found. The following two histologic subtypes have clinical relevance: - Pure fetal histology hepatoblastoma.
- Small cell undifferentiated hepatoblastoma.
Pure fetal histology hepatoblastoma Analysis of patients with initially resected hepatoblastoma tumors (before receiving chemotherapy) has suggested that patients with pure fetal histology tumors have a better prognosis than do patients with an admixture of more primitive and rapidly dividing embryonal components or other undifferentiated tissues. Studies have reported the following: - A study of patients with hepatoblastoma and pure fetal histology tumors observed the following:[41]
- The survival rate was 100% for patients who received four doses of single-agent doxorubicin. This suggested that patients with pure fetal histology tumors might not need chemotherapy after complete resection.[59,60]
- In a COG study (COG-P9645), 16 patients with pure fetal histology hepatoblastoma with two or fewer mitoses per 10 high-power fields were not treated with chemotherapy. Retrospectively, their PRETEXT groups were group I (n = 4), group II (n = 6), and group III (n = 2).[61]
- Survival was 100% with no chemotherapy given.
- All 16 patients entered on this study were alive with no evidence of disease at a median follow-up of 4.9 years (range, 9 months to 9.2 years).
Thus, complete resection of a pure fetal hepatoblastoma may preclude the need for chemotherapy. Small cell undifferentiated hepatoblastoma Small cell undifferentiated hepatoblastoma is an uncommon hepatoblastoma variant that represents a few percent of all hepatoblastomas. It tends to occur at a younger age (6-10 months) compared with other cases of hepatoblastoma [53,62] and is associated with AFP levels that are normal for age at presentation.[52,62] Histologically, small cell undifferentiated hepatoblastoma is typified by a diffuse population of small cells with scant cytoplasm resembling neuroblasts.[63] Occasional small cell undifferentiated hepatoblastomas are identical to malignant rhabdoid tumors and have the following characteristic abnormalities: - Chromosomal abnormalities. These abnormalities include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1/INI1 gene.[62,64]
- Lack of INI1. Lack of detection of INI1 by immunohistochemistry is another characteristic shared by some small cell undifferentiated hepatoblastomas and malignant rhabdoid tumors.[62]
- Poor prognosis. A third characteristic shared between small cell undifferentiated hepatoblastomas and malignant rhabdoid tumors is the poor prognosis associated with each.[53,62,65]
Patients with small cell undifferentiated hepatoblastoma whose tumors are unresectable have an especially poor prognosis.[62] Patients with stage I tumors appear to have increased risk of treatment failure when small cell elements are present.[66] For this reason, completely resected tumors composed of pure fetal histology or of mixed fetal and embryonal cells must have a thorough histologic examination as small foci of undifferentiated small cell histology indicates a need for aggressive chemotherapy.[66] Aggressive treatment for this histology is under investigation in the current COG study, COG-AHEP0731. In this study, hepatoblastoma that would otherwise be considered very low or low risk is upgraded to intermediate risk if any small cell undifferentiated elements are found (refer to Table 3 for more information). Risk Stratification There are significant differences among childhood cancer study groups in risk stratification used to determine treatment, making it difficult to compare results of the different treatments administered. Table 3 demonstrates the variability in the definitions of risk groups. Table 3. A Comparison of the Use of PRETEXT in Risk Stratification Schemes for Hepatoblastomaa,b | COG (AHEP-0731) | SIOPEL (SIOPEL-3, 3HR, 4, 6) | GPOH | JPLT (JPLT 2 and 3) |
|---|
| AFP = alpha-fetoprotein; COG = Children's Oncology Group; GPOH = Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology); JPLT = Japanese Study Group for Pediatric Liver Tumor; PRETEXT = PRE-Treatment EXTent of disease; SCU = small cell undifferentiated; SIOPEL = International Childhood Liver Tumors Strategy Group. | | a Adapted from Czauderna et al.[57] | | b Refer to Table 1for more information about the annotations used in PRETEXT. | | c The COG and PRETEXT definitions of vascular involvement differ. | | Very low risk | PRETEXT I or II; pure fetal histology; primary resection at diagnosis | | | | | Low risk/standard risk | PRETEXT I or II of any histology with primary resection at diagnosis | PRETEXT I, II, or III | PRETEXT I, II, or III | PRETEXT I, II, or III | | Intermediate riskb | PRETEXT II, III, or IV unresectable at diagnosis; or V+c, P+, E+; SCU histology | | | PRETEXT IV or any PRETEXT with rupture; or N1, P2, P2a, V3, V3a; or multifocal | | High riskb | Any PRETEXT with M+; AFP level <100 ng/mL | Any PRETEXT; V+, P+, E+, M+; SCU histology; AFP level <100 ng/mL; tumor rupture | Any PRETEXT with V+, E+, P+, M+ or multifocal | Any PRETEXT with M1 or N2; or AFP level <100 ng/mL | Treatment of Hepatoblastoma Cisplatin-based chemotherapy has resulted in a survival rate of more than 90% for children with PRETEXT AND POST-Treatment EXTent (POSTTEXT) I and II resectable disease before or after chemotherapy.[40,42,50] Chemotherapy regimens used in the treatment of hepatoblastoma and their respective outcomes are described in Table 4. (Refer to the Tumor Stratification by Imaging and Postsurgical Staging for Childhood Liver Cancer section of this summary for information describing each stage.) Table 4. Outcomes for Hepatoblastoma Multicenter Trialsa| Study | Chemotherapy Regimen | Number of Patients | Outcomes |
|---|
| AFP = alpha-fetoprotein; C5V = cisplatin, 5-fluorouracil (5FU), and vincristine; CARBO = carboplatin; CCG = Children's Cancer Group; CDDP = cisplatin; CITA = pirarubicin-cisplatin; COG = Children's Oncology Group; DOXO = doxorubicin; EFS = event-free survival; GPOH = Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology); HR = high risk; IFOS = ifosfamide; IPA = ifosfamide, cisplatin, and doxorubicin; JPLT = Japanese Study Group for Pediatric Liver Tumor; NR = not reported; OS = overall survival; PLADO = cisplatin and doxorubicin; POG = Pediatric Oncology Group; PRETEXT = PRE-Treatment EXTent of disease; SIOPEL = International Childhood Liver Tumors Strategy Group; SR = standard risk; SUPERPLADO = cisplatin, doxorubicin, and carboplatin; THP = tetrahydropyranyl-adriamycin (pirarubicin); VP = vinorelbine and cisplatin; VPE+ = venous, portal, and extrahepatic involvement; VP16 = etoposide. | | a Adapted from Czauderna et al.[57]and Meyers et al.[67] | | b Study closed early because of inferior results in the CDDP/CARBO arm. | | INT0098 (CCG/POG) 1989-1992 | C5V vs. CDDP/DOXO | Stage I/II: 50 | 4-Year EFS/OS: | | I/II = 88%/100% vs. 96%/96% | | Stage III: 83 | III = 60%/68% vs. 68%/71% | | Stage IV: 40 | IV = 14%/33% vs. 37%/42% | | P9645 (COG)b 1999-2002 | C5V vs. CDDP/CARBO | Stage I/II: Pending publication | 1-Year EFS: | | I/II: Pending publication | | Stage III: 38 | III/IV: C5V = 51%; CDDP/CARBO = 37% | | Stage IV: 50 | | HB 94 (GPOH) 1994-1997 | I/II: IFOS/CDDP/DOXO | Stage I: 27 | 4-Year EFS/OS: | | I = 89%/96% | | Stage II: 3 | II = 100%/100% | | III/IV: IFOS/CDDP/DOXO + VP/CARBO | Stage III: 25 | III = 68%/76% | | Stage IV: 14 | IV = 21%/36% | | HB 99 (GPOH) 1999-2004 | SR: IPA | SR: 58 | 3-Year EFS/OS: | | SR = 90%/88% | | HR: CARBO/VP16 | HR: 42 | HR = 52%/55% | | SIOPEL-2 1994-1998 | SR: PLADO | PRETEXT I: 6 | 3-Year EFS/OS: | | SR: 73%/91% | | PRETEXT II: 36 | | PRETEXT III: 25 | | HR: CDDP/CARBO/DOXO | PRETEXT IV: 21 | HR: IV = 48%/61% | | Metastases: 25 | HR: metastases = 36%/44% | | SIOPEL-3 1998-2006 | SR: CDDP vs. PLADO | SR: PRETEXT I: 18 | 3-Year EFS/OS: | | SR: CDDP = 83%/95%; PLADO = 85%/93% | | PRETEXT II: 133 | | PRETEXT III: 104 | | HR: SUPERPLADO | HR: PRETEXT IV: 74 | HR: Overall = 65%/69% | | VPE+: 70 | | | Metastases: 70 | Metastases = 57%/63% | | AFP <100 ng/mL: 12 | | | SIOPEL-4 2005-2009 | HR: Block A: Weekly; CDDP/3 weekly DOXO; Block B: CARBO/DOXO | PRETEXT I: 2 | 3-Year EFS/OS: | | All HR = 76%/83% | | PRETEXT II: 17 | | PRETEXT III: 27 | | PRETEXT IV: 16 | HR: IV = 75%/88% | | Metastases: 39 | HR: Metastases = 77%/79% | | JPLT 1 1991-1999 | I/II: CDDP(30)/THP-DOXO | Stage I: 9 | 5-Year EFS/OS: | | I = NR/100% | | Stage II: 32 | II = NR/76% | | III/IV: CDDP(60)/THP-DOXO | Stage IIIa: 48 | IIIa = NR/50% | | Stage IIIb: 25 | IIIb = NR/64% | | Stage IV: 20 | IV = NR/77% | | JPLT 2 1999-2010 | I: Low-dose CDDP-pirarubicin | PRETEXT I-IV: 212 | 5-Year EFS/OS: | | | I = NR/100% | | II-IV: CITA | | II = NR/89% | | | III = NR/93% | | | IV = NR/63% | | Metastases: High dose chemotherapy + stem cell transplant | | Metastases = 32% | Treatment options for newly diagnosed hepatoblastoma depend on the following: - Whether the cancer is resectable at diagnosis.
- The tumor histology.
- How the cancer responds to chemotherapy.
- Whether the cancer has metastasized.
Treatment options for hepatoblastoma that is resectable at diagnosis Approximately 20% to 30% of children with hepatoblastoma have resectable disease at diagnosis. Prognosis varies depending on the histologic subtype: - Pure fetal histology (4% of hepatoblastomas) has a 3- to 5-year OS rate of 100% with minimal or no adjuvant chemotherapy.[41,53,61]
- Non-pure fetal histology, non-small cell undifferentiated hepatoblastomas have a 3- to 4-year OS rate of 90% to 100% with adjuvant chemotherapy.[41,42,50,53,68]
- If any small cell undifferentiated elements are present, the 3-year survival rate is 40% to 70%.[52,53]
The treatment of hepatoblastoma that can be resected at diagnosis depends on the tumor histology. Treatment options for hepatoblastoma of pure fetal histology include the following: - Complete surgical resection followed by watchful waiting or chemotherapy.[61]
Evidence (complete surgical resection followed by watchful waiting or chemotherapy): - In the COG prospective clinical trial (INT0098), nine children with stage I (completely resected) pure fetal histology and fewer than two mitoses per high-power field were treated with adjuvant doxorubicin for four cycles. All nine children had 100% EFS and OS at a median follow-up of 5.1 years.[41]
- In the COG P9645 (NCT00003994) study, 16 patients with stage I (completely resected) tumor had pure fetal histology and received no adjuvant chemotherapy; the EFS and OS were 100%, including one patient who had a second surgery to address a positive tumor margin. In a retrospective PRETEXT classification of 21 of these 25 patients with adequate data, PRETEXT I, II, and III were found in seven, ten, and four patients.[61]
- Treatment of a small focus of undifferentiated small cell histology within an otherwise pure fetal histology tumor with aggressive chemotherapy has been reported in the following small series suggesting the importance of a thorough histologic examination of apparent pure fetal histology.[66] A retrospective study of 16 patients with hepatoblastoma treated at multiple institutions who had complete surgical resection, but also had elements of (or in some cases predominance of) small cell histology found in the resected tumor. Ten of 16 patients recurred, and five of these patients died of hepatoblastoma.[66]
Treatment options for hepatoblastoma of non-pure fetal histology include the following: - Gross surgical resection (with or without microscopic margins) and preoperative and/or postoperative chemotherapy.
Evidence (gross surgical resection [with or without microscopic margins] and preoperative and/or postoperative chemotherapy): - Gross surgical excision with or without microscopic margins is followed by four courses of combination chemotherapy with cisplatin, vincristine, and fluorouracil or cisplatin and doxorubicin or cisplatin alone.[40,41,42,50]
Second resection of positive margins and/or radiation therapy may not be necessary in patients with incompletely resected hepatoblastoma whose residual tumor is microscopic and who receive subsequent chemotherapy.[46,54] - In a European study conducted between 1990 and 1994, 11 patients had tumor found at the surgical margins after hepatic resection and two patients died, neither of whom had a local recurrence. None of the 11 patients underwent a second resection and only one patient received radiation therapy postoperatively. All of the patients were treated with four courses of cisplatin and doxorubicin before surgery and received two courses of postoperative chemotherapy.[46]
- In another European study of high-risk hepatoblastoma, 11 patients had microscopic residual tumor remaining after initial surgery and received two to four postoperative cycles of chemotherapy with no additional surgery. Of these 11 patients, 9 survived.[54]
- In the SIOPEL-2 study, 13 of 13 patients with microscopic positive resection margins survived.[50]
- A randomized clinical trial demonstrated comparable efficacy with cisplatin/vincristine/fluorouracil and cisplatin/doxorubicin in the treatment of hepatoblastoma.[41]
- Although outcome was nominally higher for children receiving cisplatin/doxorubicin, this difference was not statistically significant.
- The combination of cisplatin/vincristine/fluorouracil was significantly less toxic than the doses of cisplatin/doxorubicin, to which it was compared.
Results of chemotherapy clinical trials are described in Table 4. Treatment options for hepatoblastoma that is not resectable or not resected at diagnosis Tumor rupture at presentation, resulting in major hemorrhage that can be controlled by transcatheter arterial embolization or partial resection to stabilize the patient, does not preclude a favorable outcome when followed by chemotherapy and definitive surgery.[69] Treatment options for hepatoblastoma that is not resectable or is not resected at diagnosis include the following: - Chemotherapy followed by reassessment of surgical resectability and complete surgical resection.
- Chemotherapy followed by reassessment of surgical resectability and orthotopic liver transplantation.[42,44,70,71,72,73,74]
- Transarterial chemoembolization (TACE). TACE may be used to improve resectability before definitive surgical approaches.[75,76]
In recent years, almost all children with hepatoblastoma have been treated with chemotherapy, and in European centers, children with resectable hepatoblastoma are treated with preoperative chemotherapy, which may reduce the incidence of surgical complications at the time of resection.[42,46,50] Preoperative chemotherapy has been shown to be of benefit in children with hepatoblastoma. In contrast, an American intergroup study of treatment of children with hepatoblastoma encouraged resection at the time of diagnosis for all tumors amenable to resection without undue risk. The study (COG-P9645) did not treat children with stage I tumors of pure fetal histology with preoperative or postoperative chemotherapy unless they developed progressive disease.[61] In this study, most PRETEXT III and all PRETEXT IV tumors were treated with chemotherapy before resection or transplant. Patients whose tumors remain unresectable should be considered for liver transplantation.[42,44,70,71,72,73,74] In the presence of features predicting unresectability, early coordination with a pediatric liver transplant service is critical.[51] Evidence (chemotherapy followed by reassessment of surgical resectability and complete surgical resection): - In a SIOPEL study (SIOPEL-1), preoperative chemotherapy (doxorubicin and cisplatin) was given to all children with hepatoblastoma with or without metastases. The chemotherapy was well tolerated. After chemotherapy, and excluding those who received liver transplant (less than 5% of patients), complete resection was performed.[42]
- Complete resection was obtained in 87% of children.
- This strategy resulted in an OS of 75% at 5 years after diagnosis.
- Identical results were seen in a follow-up international study (SIOPEL-2).[50]
- SIOPEL compared cisplatin alone with cisplatin and doxorubicin in patients with preoperative standard-risk hepatoblastoma. Standard risk was defined as tumor confined to the liver and not involving more than three sectors.[68][Level of evidence:1iiA]
- The rates of resection were similar for the cisplatin (95%) and cisplatin/doxorubicin (93%) groups.
- The OS rates were also similar for the cisplatin (95%) and cisplatin/doxorubicin (93%) groups.
- In a pilot study, SIOPEL-3HR, cisplatin alternating with carboplatin/doxorubicin was administered in a dose intensive fashion to high-risk patients with hepatoblastoma.[54]
- In 74 patients with PRETEXT IV tumors, 22 of whom also had metastases, 31 became resectable and 26 underwent transplant. The 3-year OS of this group was 69% ± 11%.
- Of the 70 patients with metastases enrolled in the trial, the 3-year EFS rate was 56% and the OS rate was 62%. Of patients with lung metastases, 50% were able to achieve complete remission of metastases with chemotherapy alone (without lung surgery).
- In SIOPEL-4, cisplatin was dose-intensified (timing, every 2 weeks) in a single-arm prospective study. Three-year EFS was 76% and OS was 83%. Toxicity was significant but acceptable.[47][Level of evidence: 2A]
- In approximately 75% of children and adolescents with initially unresectable hepatoblastoma, tumors can be rendered resectable with cisplatin-based preoperative chemotherapy, and 60% to 65% will survive disease-free.[77]
- A combination of ifosfamide, cisplatin, and doxorubicin followed by postinduction resection has also been used in the treatment of advanced-stage disease.[78]
- In the United States, unresectable tumors have been treated with chemotherapy before resection or transplant.[39,40,41,61] Based on radiographical imaging, most of stage III and IV hepatoblastomas are rendered resectable after two cycles of chemotherapy.[79]
Chemotherapy followed by TACE followed by high-intensity focused ultrasound showed promising results in China for PRETEXT III and IV patients with hepatoblastoma, some of whom were resectable but did not undergo surgical resection because of parent refusal.[80] Treatment options for hepatoblastoma with metastases at diagnosis The outcome for metastatic hepatoblastoma at diagnosis is poor, but long-term survival and cure is possible.[39,40,41] Survival rates at 3 to 5 years range from 20% to 60%.[54,81,82] Treatment options for hepatoblastoma with metastases at diagnosis include the following: - Chemotherapy followed by reassessment of surgical resectability.
- If the primary tumor and extrahepatic disease is resectable after chemotherapy, surgical resection followed by additional chemotherapy.
- If extrahepatic disease is in complete remission after chemotherapy and/or surgery, but the primary tumor remains unresectable, orthotopic liver transplantation.
- If extrahepatic disease is not resectable or the patient is not a transplant candidate, additional chemotherapy, TACE, or radiation therapy.
The standard combination chemotherapy regimen is four courses of cisplatin/vincristine/fluorouracil [41] or doxorubicin/cisplatin [42,61,81] followed by attempted complete tumor resection. If the tumor is completely removed, two postoperative courses of the same chemotherapy are usually given. Study results for different chemotherapy regimens have been reported (refer to Table 4 for more information). High-dose chemotherapy with stem cell rescue does not appear to be more effective than standard multiagent chemotherapy.[83] Evidence (chemotherapy followed by reassessment of surgical resectability; complete surgical resection of the primary tumor and extrahepatic disease followed by additional chemotherapy): - The SIOPEL-1 study employed a well-tolerated regimen of doxorubicin/cisplatin chemotherapy.[42]
- About 50% of patients with metastases at presentation survived 5 years after diagnosis. Half of these survivors developed progressive disease that was successfully treated with surgery and other interventions.
- In rare cases, chemotherapy has eradicated pulmonary metastases and eliminated multinodular tumor foci in the liver. In the SIOPEL-3HR study, patients with metastatic disease were treated with intensive platinum- and doxorubicin-based multidrug chemotherapy.[54]
- This regimen induced complete regression in approximately 50% of patients, with subsequent 3-year EFS of 56%.
- A prospective feasibility trial (SIOPEL-4 [NCT00077389]) of dose-dense, cisplatin-based chemotherapy and radical surgery evaluated 62 patients with high-risk hepatoblastoma.[47][Level of evidence: 3iiDi]
- This treatment regimen resulted in a 3-year EFS of 76% and 3-year OS of 83%.
- Of 37 patients with distant metastases on the study, 27 (78%) were disease free at 3 years.
- A randomized clinical trial compared cisplatin/vincristine/fluorouracil with cisplatin/doxorubicin. Although outcome was nominally better for children receiving cisplatin/doxorubicin, this difference was not statistically significant, and the combination of cisplatin/vincristine/fluorouracil was less toxic than the regimen of cisplatin/doxorubicin.[41]
- The regimen of cisplatin/doxorubicin used in the international studies appears to be less toxic than that used in the North American study.[42]
- Addition of carboplatin to intensify the cisplatin/doxorubicin did not increase its efficacy in SIOPEL-2.[50]
- A regimen of intensified platinum therapy with alternating cisplatin and carboplatin in COG study P9605 was associated with a poorer EFS outcome.[84]
- A combination of ifosfamide, cisplatin, and doxorubicin has been used in the treatment of advanced-stage disease.[78]
- In a COG study (AHEP0731 [NCT00980460]) that included 30 children with metastatic hepatoblastoma, a therapeutic window of two cycles of vincristine and irinotecan (VI) was administered before standard cisplatin-based therapy to assess the activity of this two-drug combination in hepatoblastoma.[85]
- Among the 30 patients, nine patients met criteria for a Response Evaluation Criteria In Solid Tumors (RECIST) objective response and 11 patients met criteria for an AFP response (defined as a one-log decrease in AFP level), with 14 patients meeting the criteria for one or the other response criteria. Additional clinical trials are needed to define the role of the VI combination for the treatment of hepatoblastoma.
In patients with resected primary tumor, any remaining pulmonary metastasis is surgically removed, if possible.[81] A review of patients treated on a U.S. intergroup trial suggested that resection of metastasis may be done at the time of resection of the primary tumor.[82][Level of evidence: 3iiA] If extrahepatic disease is in complete remission after chemotherapy, and the primary tumor remains unresectable, an orthotopic liver transplantation may be performed.[47,54,61,78] The outcome results are discrepant for patients with lung metastases at diagnosis who undergo orthotopic liver transplantation after complete resolution of lung disease in response to pretransplant chemotherapy. Some studies have reported favorable outcomes for these groups,[47,54,74,78] while others have noted high rates of hepatoblastoma recurrence.[44,70,73,75] All of these studies are limited by small patient numbers; further study is needed to better define outcomes for this subset of patients. If extrahepatic disease is not resectable after chemotherapy or the patient is not a transplant candidate, alternative treatment approaches include the following: - Nonstandard chemotherapy agents. Nonstandard chemotherapy agents, such as irinotecan, high-dose cisplatin/etoposide, or continuous-infusion doxorubicin have been used.[86,87,88]; [89][Level of evidence: 3iiA]
- Transarterial chemoembolization.[76,90]
- Radiation therapy.[91]
Treatment options under clinical evaluation for newly diagnosed hepatoblastoma The following is an example of a national and/or institutional clinical trial that is currently being conducted. Information about ongoing clinical trials is available from the NCI website. - COG-AHEP0731 (Combination Chemotherapy in Treating Young Patients With Newly Diagnosed Liver Cancer): Complete surgical resection is attempted for all PRETEXT I tumors and those PRETEXT II tumors with greater than 1 cm radiographic margin on the middle hepatic vein, the retrohepatic inferior vena cava, and the portal bifurcation. PRETEXT I pure fetal histology with an AFP level greater than 100 ng/mL, non-small cell undifferentiated (very low risk) is treated with resection and observation with no chemotherapy.
All patients with metastatic hepatoblastoma and patients with any stage or PRETEXT group of hepatoblastoma and initial AFP less than 100 ng/mL are treated with the novel combination of vincristine, irinotecan, and temsirolimus (VIT) to estimate the response rate of this new combination of agents. This regimen includes two cycles of up-front VIT in the initial 6 weeks of therapy. Patients who respond to VIT will continue to receive this combination. Responding patients will receive a total of six cycles of cisplatin, 5-flouorouracil, and vincristine (C5VD) therapy with two more cycles of VIT (total of four). Nonresponding patients will receive only the six cycles of C5VD after the up-front window therapy.
Treatment options for progressive or recurrent hepatoblastoma The prognosis for a patient with recurrent or progressive hepatoblastoma depends on several factors, including the following:[92] - Site of recurrence.
- Previous treatment.
- Individual patient considerations.
Treatment options for recurrent or progressive hepatoblastoma include the following: - Surgical resection. In patients with hepatoblastoma completely resected at initial diagnosis, aggressive surgical treatment of isolated pulmonary metastases that develop in the course of the disease may make extended disease-free survival possible.[82,92]
If possible, isolated metastases is resected completely in patients whose primary tumor is controlled.[93] A retrospective study of patients in SIOPEL studies 1, 2, and 3 showed a 12% incidence of recurrence after complete remission by imaging and AFP. Outcome after recurrence was best if the tumor was amenable to surgery. Of patients who underwent chemotherapy and surgery, 3-year EFS was 34% and OS was 43%.[92][Level of evidence: 3iiA] Treatment in a clinical trial should be considered if all of the recurrent disease cannot be surgically removed. Phase I and phase II clinical trials may be appropriate and should be considered. - Chemotherapy. Analysis of survival after recurrence demonstrated that some patients treated with cisplatin/vincristine/fluorouracil could be salvaged with doxorubicin-containing regimens, but patients treated with doxorubicin/cisplatin could not be salvaged with vincristine/fluorouracil.[94] Addition of doxorubicin to vincristine/fluorouracil/cisplatin is under clinical evaluation in the COG study COG-AHEP0731. Combined vincristine/irinotecan and single-agent irinotecan have been used with some success.[89]; [88][Level of evidence: 3iiiA]
- Liver transplantation. Liver transplantation should be considered for patients with nonmetastatic disease recurrence in the liver that is not amenable to resection.[44,70,73]
- Percutaneous ablation techniques may also be considered.[95]
References:
-
Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, Md: National Cancer Institute, 2012, Section 29. Also available online. Last accessed May 31, 2017.
-
Bulterys M, Goodman MT, Smith MA, et al.: Hepatic tumors. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 91-98. Also available online. Last accessed April 13, 2017.
-
Turcotte LM, Georgieff MK, Ross JA, et al.: Neonatal medical exposures and characteristics of low birth weight hepatoblastoma cases: a report from the Children's Oncology Group. Pediatr Blood Cancer 61 (11): 2018-23, 2014.
-
Tanimura M, Matsui I, Abe J, et al.: Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res 58 (14): 3032-5, 1998.
-
McLaughlin CC, Baptiste MS, Schymura MJ, et al.: Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol 163 (9): 818-28, 2006.
-
Darbari A, Sabin KM, Shapiro CN, et al.: Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 38 (3): 560-6, 2003.
-
Kamien BA, Gabbett MT: Aicardi syndrome associated with hepatoblastoma and pulmonary sequestration. Am J Med Genet A 149A (8): 1850-2, 2009.
-
DeBaun MR, Tucker MA: Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr 132 (3 Pt 1): 398-400, 1998.
-
Weksberg R, Shuman C, Smith AC: Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 137C (1): 12-23, 2005.
-
Iwama T, Mishima Y: Mortality in young first-degree relatives of patients with familial adenomatous polyposis. Cancer 73 (8): 2065-8, 1994.
-
Garber JE, Li FP, Kingston JE, et al.: Hepatoblastoma and familial adenomatous polyposis. J Natl Cancer Inst 80 (20): 1626-8, 1988.
-
Giardiello FM, Petersen GM, Brensinger JD, et al.: Hepatoblastoma and APC gene mutation in familial adenomatous polyposis. Gut 39 (96): 867-9, 1996.
-
Ito E, Sato Y, Kawauchi K, et al.: Type 1a glycogen storage disease with hepatoblastoma in siblings. Cancer 59 (10): 1776-80, 1987.
-
Ikeda H, Hachitanda Y, Tanimura M, et al.: Development of unfavorable hepatoblastoma in children of very low birth weight: results of a surgical and pathologic review. Cancer 82 (9): 1789-96, 1998.
-
Spector LG, Birch J: The epidemiology of hepatoblastoma. Pediatr Blood Cancer 59 (5): 776-9, 2012.
-
Buonuomo PS, Ruggiero A, Vasta I, et al.: Second case of hepatoblastoma in a young patient with Simpson-Golabi-Behmel syndrome. Pediatr Hematol Oncol 22 (7): 623-8, 2005 Oct-Nov.
-
Tan ZH, Lai A, Chen CK, et al.: Association of trisomy 18 with hepatoblastoma and its implications. Eur J Pediatr 173 (12): 1595-8, 2014.
-
Trobaugh-Lotrario AD, Venkatramani R, Feusner JH: Hepatoblastoma in children with Beckwith-Wiedemann syndrome: does it warrant different treatment? J Pediatr Hematol Oncol 36 (5): 369-73, 2014.
-
Hoyme HE, Seaver LH, Jones KL, et al.: Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet 79 (4): 274-8, 1998.
-
Clericuzio CL, Martin RA: Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 11 (3): 220-2, 2009.
-
Algar EM, St Heaps L, Darmanian A, et al.: Paternally inherited submicroscopic duplication at 11p15.5 implicates insulin-like growth factor II in overgrowth and Wilms' tumorigenesis. Cancer Res 67 (5): 2360-5, 2007.
-
Steenman M, Westerveld A, Mannens M: Genetics of Beckwith-Wiedemann syndrome-associated tumors: common genetic pathways. Genes Chromosomes Cancer 28 (1): 1-13, 2000.
-
Albrecht S, Hartmann W, Houshdaran F, et al.: Allelic loss but absence of mutations in the polyspecific transporter gene BWR1A on 11p15.5 in hepatoblastoma. Int J Cancer 111 (4): 627-32, 2004.
-
Clericuzio CL, Chen E, McNeil DE, et al.: Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J Pediatr 143 (2): 270-2, 2003.
-
Aretz S, Koch A, Uhlhaas S, et al.: Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr Blood Cancer 47 (6): 811-8, 2006.
-
Hirschman BA, Pollock BH, Tomlinson GE: The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J Pediatr 147 (2): 263-6, 2005.
-
Koch A, Denkhaus D, Albrecht S, et al.: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 59 (2): 269-73, 1999.
-
Yoon JM, Burns RC, Malogolowkin MH, et al.: Treatment of infantile choriocarcinoma of the liver. Pediatr Blood Cancer 49 (1): 99-102, 2007.
-
Boman F, Bossard C, Fabre M, et al.: Mesenchymal hamartomas of the liver may be associated with increased serum alpha foetoprotein concentrations and mimic hepatoblastomas. Eur J Pediatr Surg 14 (1): 63-6, 2004.
-
Blohm ME, Vesterling-Hörner D, Calaminus G, et al.: Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 15 (2): 135-42, 1998 Mar-Apr.
-
Perilongo G, Malogolowkin M, Feusner J: Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer 59 (5): 818-21, 2012.
-
Trobaugh-Lotrario AD, Katzenstein HM: Chemotherapeutic approaches for newly diagnosed hepatoblastoma: past, present, and future strategies. Pediatr Blood Cancer 59 (5): 809-12, 2012.
-
Trobaugh-Lotrario AD, Chaiyachati BH, Meyers RL, et al.: Outcomes for patients with congenital hepatoblastoma. Pediatr Blood Cancer 60 (11): 1817-25, 2013.
-
Fuchs J, Rydzynski J, Von Schweinitz D, et al.: Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer 95 (1): 172-82, 2002.
-
Aronson DC, Schnater JM, Staalman CR, et al.: Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 23 (6): 1245-52, 2005.
-
Czauderna P, Haeberle B, Hiyama E, et al.: The Children's Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 52: 92-101, 2016.
-
Meyers RL, Maibach R, Hiyama E, et al.: Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol 18 (1): 122-131, 2017.
-
Becker K, Furch C, Schmid I, et al.: Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr Blood Cancer 62 (1): 24-8, 2015.
-
Ortega JA, Krailo MD, Haas JE, et al.: Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (12): 2167-76, 1991.
-
Douglass EC, Reynolds M, Finegold M, et al.: Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol 11 (1): 96-9, 1993.
-
Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000.
-
Pritchard J, Brown J, Shafford E, et al.: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18 (22): 3819-28, 2000.
-
Meyers RL, Czauderna P, Otte JB: Surgical treatment of hepatoblastoma. Pediatr Blood Cancer 59 (5): 800-8, 2012.
-
Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
-
McAteer JP, Goldin AB, Healey PJ, et al.: Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 17 (8): 744-50, 2013.
-
Schnater JM, Aronson DC, Plaschkes J, et al.: Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 94 (4): 1111-20, 2002.
-
Zsiros J, Brugieres L, Brock P, et al.: Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14 (9): 834-42, 2013.
-
Van Tornout JM, Buckley JD, Quinn JJ, et al.: Timing and magnitude of decline in alpha-fetoprotein levels in treated children with unresectable or metastatic hepatoblastoma are predictors of outcome: a report from the Children's Cancer Group. J Clin Oncol 15 (3): 1190-7, 1997.
-
Brown J, Perilongo G, Shafford E, et al.: Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36 (11): 1418-25, 2000.
-
Perilongo G, Shafford E, Maibach R, et al.: Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 40 (3): 411-21, 2004.
-
D'Antiga L, Vallortigara F, Cillo U, et al.: Features predicting unresectability in hepatoblastoma. Cancer 110 (5): 1050-8, 2007.
-
De Ioris M, Brugieres L, Zimmermann A, et al.: Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer 44 (4): 545-50, 2008.
-
Meyers RL, Rowland JR, Krailo M, et al.: Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 53 (6): 1016-22, 2009.
-
Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010.
-
Schneider DT, Calaminus G, Göbel U: Diagnostic value of alpha 1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr Hematol Oncol 18 (1): 11-26, 2001 Jan-Feb.
-
Nakagawara A, Ikeda K, Tsuneyoshi M, et al.: Hepatoblastoma producing both alpha-fetoprotein and human chorionic gonadotropin. Clinicopathologic analysis of four cases and a review of the literature. Cancer 56 (7): 1636-42, 1985.
-
Czauderna P, Lopez-Terrada D, Hiyama E, et al.: Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 26 (1): 19-28, 2014.
-
López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
-
Weinberg AG, Finegold MJ: Primary hepatic tumors of childhood. Hum Pathol 14 (6): 512-37, 1983.
-
Haas JE, Muczynski KA, Krailo M, et al.: Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer 64 (5): 1082-95, 1989.
-
Malogolowkin MH, Katzenstein HM, Meyers RL, et al.: Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol 29 (24): 3301-6, 2011.
-
Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, et al.: Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer 52 (3): 328-34, 2009.
-
Rowland JM: Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol 39 (5): 478-83, 2002.
-
Gunawan B, Schäfer KL, Sattler B, et al.: Undifferentiated small cell hepatoblastoma with a chromosomal translocation t(22;22)(q11;q13). Histopathology 40 (5): 485-7, 2002.
-
Conran RM, Hitchcock CL, Waclawiw MA, et al.: Hepatoblastoma: the prognostic significance of histologic type. Pediatr Pathol 12 (2): 167-83, 1992 Mar-Apr.
-
Haas JE, Feusner JH, Finegold MJ: Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer 92 (12): 3130-4, 2001.
-
Meyers RL, Tiao G, de Ville de Goyet J, et al.: Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 26 (1): 29-36, 2014.
-
Perilongo G, Maibach R, Shafford E, et al.: Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 361 (17): 1662-70, 2009.
-
Madanur MA, Battula N, Davenport M, et al.: Staged resection for a ruptured hepatoblastoma: a 6-year follow-up. Pediatr Surg Int 23 (6): 609-11, 2007.
-
Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
-
Molmenti EP, Wilkinson K, Molmenti H, et al.: Treatment of unresectable hepatoblastoma with liver transplantation in the pediatric population. Am J Transplant 2 (6): 535-8, 2002.
-
Czauderna P, Otte JB, Aronson DC, et al.: Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer 41 (7): 1031-6, 2005.
-
Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006.
-
Pham TA, Gallo AM, Concepcion W, et al.: Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg 150 (12): 1150-8, 2015.
-
Xianliang H, Jianhong L, Xuewu J, et al.: Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol 26 (1): 60-3, 2004.
-
Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
-
Reynolds M, Douglass EC, Finegold M, et al.: Chemotherapy can convert unresectable hepatoblastoma. J Pediatr Surg 27 (8): 1080-3; discussion 1083-4, 1992.
-
von Schweinitz D, Hecker H, Harms D, et al.: Complete resection before development of drug resistance is essential for survival from advanced hepatoblastoma--a report from the German Cooperative Pediatric Liver Tumor Study HB-89. J Pediatr Surg 30 (6): 845-52, 1995.
-
Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
-
Wang S, Yang C, Zhang J, et al.: First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 59 (1): 170-7, 2014.
-
Perilongo G, Brown J, Shafford E, et al.: Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 89 (8): 1845-53, 2000.
-
Meyers RL, Katzenstein HM, Krailo M, et al.: Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42 (12): 2050-6, 2007.
-
Karski EE, Dvorak CC, Leung W, et al.: Treatment of hepatoblastoma with high-dose chemotherapy and stem cell rescue: the pediatric blood and marrow transplant consortium experience and review of the literature. J Pediatr Hematol Oncol 36 (5): 362-8, 2014.
-
Malogolowkin MH, Katzenstein H, Krailo MD, et al.: Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol 24 (18): 2879-84, 2006.
-
Katzenstein HM, Furman WL, Malogolowkin MH, et al.: Upfront window vincristine/irinotecan treatment of high-risk hepatoblastoma: A report from the Children's Oncology Group AHEP0731 study committee. Cancer : , 2017.
-
Katzenstein HM, Rigsby C, Shaw PH, et al.: Novel therapeutic approaches in the treatment of children with hepatoblastoma. J Pediatr Hematol Oncol 24 (9): 751-5, 2002.
-
Palmer RD, Williams DM: Dramatic response of multiply relapsed hepatoblastoma to irinotecan (CPT-11). Med Pediatr Oncol 41 (1): 78-80, 2003.
-
Qayed M, Powell C, Morgan ER, et al.: Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer 54 (5): 761-3, 2010.
-
Zsíros J, Brugières L, Brock P, et al.: Efficacy of irinotecan single drug treatment in children with refractory or recurrent hepatoblastoma--a phase II trial of the childhood liver tumour strategy group (SIOPEL). Eur J Cancer 48 (18): 3456-64, 2012.
-
Sue K, Ikeda K, Nakagawara A, et al.: Intrahepatic arterial injections of cisplatin-phosphatidylcholine-Lipiodol suspension in two unresectable hepatoblastoma cases. Med Pediatr Oncol 17 (6): 496-500, 1989.
-
Habrand JL, Nehme D, Kalifa C, et al.: Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23 (3): 525-31, 1992.
-
Semeraro M, Branchereau S, Maibach R, et al.: Relapses in hepatoblastoma patients: clinical characteristics and outcome--experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer 49 (4): 915-22, 2013.
-
Matsunaga T, Sasaki F, Ohira M, et al.: Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int 19 (3): 142-6, 2003.
-
Malogolowkin MH, Katzenstein HM, Krailo M, et al.: Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol 26 (14): 2379-83, 2008.
-
Liu B, Zhou L, Huang G, et al.: First Experience of Ultrasound-guided Percutaneous Ablation for Recurrent Hepatoblastoma after Liver Resection in Children. Sci Rep 5: 16805, 2015.
Hepatocellular CarcinomaIncidence The annual incidence of hepatocellular carcinoma in the United States is 0.8 per 1 million children between the ages of 0 and 14 years and 1.5 per 1 million adolescents aged 15 to 19 years.[1] Although the incidence of hepatocellular carcinoma in adults in the United States has steadily increased since the 1970s, possibly because of the increased frequency of chronic hepatitis C infection,[2] the incidence in children has not increased. In several Asian countries, the incidence of hepatocellular carcinoma in children is 10 times higher than that in North America. The high incidence appears to be related to the incidence of perinatally acquired hepatitis B, which can be prevented in most cases by vaccination and administration of hepatitis B immune globulin to the newborn.[3] Fibrolamellar hepatocellular carcinoma, a subtype of hepatocellular carcinoma that is unrelated to cirrhosis, hepatitis B virus (HBV), or hepatitis C virus (HCV) infection, generally occurs in adolescents and young adults, but has been reported in infants.[4] Risk factors Conditions associated with hepatocellular carcinoma are described in Table 5. Table 5. Conditions Associated With Hepatocellular Carcinoma| Associated Disorder | Clinical Findings |
|---|
| Alagille syndrome[5] | Broad prominent forehead, deep set eyes, and small prominent chin. Abnormality of bile ducts leads to intrahepatic scarring. | | Glycogen storage diseases I-IV[6] | Symptoms vary by individual disorder. | | Hepatitis B and C[7,8,9] | Refer to the Hepatitis B and hepatitis C infectionsection of this summary for more information. | | Progressive familial intrahepatic cholestasis[10,11] | Symptoms of jaundice, pruritus, and failure to thrive begin in infancy and progress to portal hypertension and liver failure. | | Tyrosinemia[12] | First few months of life: failure to thrive, vomiting, jaundice. | Alagille syndrome Alagille syndrome is an autosomal dominant genetic syndrome involving the bile ducts of the liver with a characteristic facies. It also often involves the heart and blood vessels in the brain and kidney. It is usually caused by mutation in or deletion of the JAG1 gene.[5] Hepatitis B and hepatitis C infection In children, hepatocellular carcinoma is associated with perinatally acquired HBV, whereas in adults it is associated with chronic HBV and HCV infection.[7,8,9] Widespread hepatitis B immunization has decreased the incidence of hepatocellular carcinoma in Asia.[3] Compared with adults, the incubation period from hepatitis virus infection to the genesis of hepatocellular carcinoma is extremely short in a small subset of children with perinatally acquired virus. Mutations in the met/hepatocyte growth factor receptor gene could be one mechanism that results in a shortened incubation period.[13] Hepatitis C infection is associated with development of cirrhosis and hepatocellular carcinoma that takes decades to develop and is generally not seen in children.[9] Cirrhosis in children, compared with cirrhosis in adults, is much less commonly involved in the development of hepatocellular carcinoma, and is found in only 20% to 35% of children with hepatocellular carcinoma tumors. Nonviral liver injury Specific types of nonviral liver injury and cirrhosis that are associated with hepatocellular carcinoma in children include the following: - Tyrosinemia. Tyrosinemia patients are regularly screened for hepatocellular carcinoma, even if they are treated with nitisinone.[12] Nitisinone can prevent cirrhosis and decrease the incidence of hepatocellular carcinoma, especially when administered during infancy, after neonatal screening is used to diagnose tyrosinemia.[14] As of 2014, only a minority of state screening programs had adopted a highly recommended, new, more predictive newborn screen that is much more effective in newborns aged 24 to 48 hours.[15]
In an Iranian study, 36 children underwent liver transplantation for tyrosinemia.[16] Twenty-two children had liver nodules greater than 10 cm, and in 20 children, the nodules were cirrhotic. Median age at transplant was 3.9 years. Five of 19 children older than 2 years had hepatocellular cancer, and no children younger than 2 years had hepatocellular cancer in the resected liver. - Aggressive familial intrahepatic cholestasis. Hepatocellular carcinoma may also arise in very young children with mutations in the bile salt export pump ABCB11, which causes progressive familial intrahepatic cholestasis.[10]
Diagnosis Refer to the Diagnosis subsection in the Hepatoblastoma section of this summary for more information. Prognosis and Prognostic Factors The 5-year overall survival (OS) rate is 42% for children and adolescents with hepatocellular carcinoma.[1] The 5-year survival for hepatocellular carcinoma may be dependent on stage; in an intergroup chemotherapy study conducted in the 1990s, seven of eight stage I patients survived and less than 10% of stage III and IV patients survived.[1,17] An analysis of Surveillance, Epidemiology, and End Results (SEER) data found a 5-year OS rate of 24%, 10-year rate of 23%, and 20-year rate of 8% in patients aged up to 19 years, suggesting improved outcome related to more recent treatment. In a multivariate analysis of the SEER data, surgical resection, localized tumor, and non-Hispanic ethnicity all had improved outcome. Complete surgical resection versus incomplete resection was associated with 60% versus 0% OS.[18][Level of evidence: 3iiiA] Factors affecting prognosis include the following: - Treatment-related factors:
Cure of hepatocellular carcinoma requires gross tumor resection. However, hepatocellular carcinoma is often extensively invasive or multicentric, and less than 30% are resectable. Orthotopic liver transplantation has been successful in selected children with hepatocellular carcinoma.[19] - PRE-Treatment EXTent of disease (PRETEXT) group (resectability) is also a prognostic factor (refer to the Risk Stratification section of this summary for more information).
- Tumor histology:
Refer to the Histology section of this summary for more information.
Histology The cells of hepatocellular carcinoma are epithelial in appearance. Hepatocellular carcinoma commonly arises in the right lobe of the liver. Fibrolamellar carcinoma A distinctive histologic variant of hepatocellular carcinoma, termed fibrolamellar carcinoma, has been described in the livers of older children and young adults and, rarely, in infants.[4,20] This histology is characterized by a fusion transcript created by deletion of a 400 kb section of chromosome 19, which was found in 15 of 15 tumors that were tested.[21] Fibrolamellar carcinoma is thought to be associated with an improved prognosis and is not associated with cirrhosis.[2,20,22] Unlike nonfibrolamellar hepatocellular carcinoma in adults, fibrolamellar hepatocellular carcinoma in older children and adults is not clearly increasing in incidence over time.[2,20] The improved outcome in older studies may be related to a higher proportion of tumors being less invasive and more resectable in the absence of cirrhosis; the outcome in recent prospective studies, when compared stage for stage and PRETEXT group to PRETEXT group, is not different from hepatocellular carcinomas.[23,24]; [25][Level of evidence: 3iiA] Hepatocellular carcinoma, not otherwise specified (NOS) Hepatocellular carcinoma, NOS is also known as transitional liver cell tumor. This tumor with characteristics of both hepatoblastoma and hepatocellular carcinoma is a rare neoplasm that is found in older children and adolescents, and has a putative intermediate position between hepatoblasts and more mature hepatocyte-like tumor cells. The tumor cells may vary in regions of the tumor between classical hepatoblastoma and obvious hepatocellular carcinoma. In the international consensus classification, these tumors are referred to as hepatocellular carcinoma, NOS.[26] The tumors are usually unifocal and may have central necrosis at presentation. Response to chemotherapy has not been rigorously studied but is felt to be much like hepatocellular carcinoma.[27] Treatment of Hepatocellular Carcinoma Treatment options for newly diagnosed hepatocellular carcinoma depend on the following: - Whether the cancer is resectable at diagnosis.
- How the cancer responds to chemotherapy.
- Whether the cancer has metastasized.
- Whether the cancer is HBV-related.
Treatment options for hepatocellular carcinoma that is resectable at diagnosis Treatment options for hepatocellular carcinoma that is resectable at diagnosis include the following: - Complete surgical resection of the primary tumor followed by chemotherapy.
- Chemotherapy followed by complete surgical resection of the primary tumor.[23]
- Complete surgical resection without chemotherapy.
Surgical resection and chemotherapy are the mainstays of treatment for resectable hepatocellular carcinoma. Evidence (surgical resection followed by chemotherapy): - Seven of eight patients with stage I hepatocellular carcinoma who were given adjuvant cisplatin-based chemotherapy survived disease free.[17]
- In a survey of childhood liver tumors treated before the consistent use of chemotherapy, only 12 of 33 patients with hepatocellular carcinoma who had complete excision of the tumor survived.[28] This suggests that adjuvant chemotherapy may benefit children with completely resected hepatocellular carcinoma.
- Cisplatin and doxorubicin may be administered as adjuvant therapy since these agents are active in the treatment of hepatocellular carcinoma.[23]
- In an analysis of SEER data for children and adolescents younger than 20 years diagnosed between 1976 and 2009, those undergoing complete resection had a 60% 5-year OS and those who did not have a complete resection had a 0% 5-year OS.[18][Level of evidence: 3iiiA]
Despite improvements in surgical techniques, chemotherapy delivery, and patient supportive care in the past 20 years, clinical trials of cancer chemotherapy for childhood hepatocellular carcinoma have not shown improved survival.[23] The International Childhood Liver Tumors Strategy Group (SIOPEL) studies in Europe have observed no improvement in 5-year OS since 1990. The only long-term survivors were patients whose tumors were resectable at diagnosis, which was less than 30% of children entered in the study.[29] However, some liver transplant studies (complete resection with transplant with or without neoadjuvant chemotherapy) have shown OS that is superior to the SIOPEL studies.[30,31,32,33] Treatment options for nonmetastatic hepatocellular carcinoma that is not resectable at diagnosis The use of neoadjuvant chemotherapy or transarterial chemoembolization (TACE) to enhance resectability or liver transplant, which may result in complete resection of tumor, is necessary for cure. Treatment options for nonmetastatic hepatocellular carcinoma that is not resectable at diagnosis include the following: - Chemotherapy followed by reassessment of surgical resectability. If the primary tumor is resectable, complete surgical resection.
- Chemotherapy followed by reassessment of surgical resectability. If the primary tumor is unresectable:
- Orthotopic liver transplantation.
- Temporizing TACE followed by complete resection or liver transplant.
- TACE alone.
Evidence (chemotherapy followed by reassessment of surgical resectability and complete surgical resection of the primary tumor): - A prospective study of 41 patients who were to receive preoperative cisplatin/doxorubicin chemotherapy resulted in some degree of decrease in tumor size, with a decrease in alpha-fetoprotein (AFP) levels in about 50% of patients. The responders had a superior tumor resectability and survival, although the OS was 28% and only those undergoing complete resection survived.[23]
Evidence (chemotherapy or TACE followed by reassessment of surgical resectability; treatment options for unresectable primary tumor after chemotherapy or TACE): - Patients whose primary tumor remains unresectable after chemotherapy should be considered for orthotopic liver transplantation. Liver transplantation has been a successful therapy for children with unresectable hepatocellular carcinoma; survival is about 60%, with most deaths resulting from tumor recurrence.[19,33,34,35,36]
- A review of treatment for hepatocellular carcinoma in patients younger than 20 years reported to SEER revealed that 75% of patients underwent resection and 25% underwent liver transplantation. The 5-year OS was 53.4% with resection and 85.3% with transplantation, suggesting the criteria for transplantation in hepatocellular carcinoma might be liberalized for overall patient benefit. This approach would benefit from prospective testing.[37]
- TACE followed by complete surgical resection of primary tumor may be an alternative to the use of surgical resection after chemotherapy. Studies in adults in China suggest that repeated hepatic TACE before surgery may improve the outcome of subsequent hepatectomy.[38] A meta-analysis found seven randomized trials that compared resection alone versus TACE followed by resection. The 3-year event-free survival (EFS) and OS showed no difference between the two groups, but the 5-year EFS and OS favored TACE followed by resection.[39]
If the primary tumor is not resectable after chemotherapy and the patient is not a transplant candidate, alternative treatment approaches used in adults include the following: - Sorafenib.
- TACE.
- Cryosurgery.
- Intratumoral injection of alcohol.
- Radiation therapy.
There is little or no data on the use of these alternative treatment approaches in children. Limited data from a European pilot study suggest that sorafenib was well tolerated in 12 newly diagnosed children and adolescents with advanced hepatocellular carcinoma when given in combination with standard chemotherapy with cisplatin and doxorubicin.[40] Further study is needed to define its role in the treatment of children with hepatocellular carcinoma. Cryosurgery, intratumoral injection of alcohol, and radiofrequency ablation can successfully treat small (<5 cm) tumors in adults with cirrhotic livers.[38,41,42] Some local approaches such as cryosurgery, radiofrequency ablation, and TACE that suppress hepatocellular carcinoma tumor progression are used as bridging therapy in adults to delay tumor growth while on a waiting list for cadaveric liver transplant.[43] (Refer to the PDQ summary on Adult Primary Liver Cancer Treatment for more information.) Treatment options for hepatocellular carcinoma with metastases at diagnosis No specific treatment has proven effective for metastatic hepatocellular carcinoma in the pediatric age group. In two prospective trials, cisplatin plus either vincristine/fluorouracil or continuous infusion doxorubicin was ineffective in adequately treating 25 patients with metastatic hepatocellular carcinoma.[17,23] Occasional patients may transiently benefit from treatment with cisplatin/doxorubicin therapy, especially if localized hepatic tumor shrinks adequately to allow resection of disease and metastases disappear or become resectable. Treatment options for hepatitis B virus (HBV)-related hepatocellular carcinoma Although HBV-related hepatocellular carcinoma is not common in children in the United States, nucleotide/nucleoside analog HBV inhibitor treatment improves postoperative prognosis in children and adults treated in China.[44] Treatment options for HBV-related hepatocellular carcinoma include the following: - Antiviral therapy.
Evidence (antiviral therapy): - In a randomized controlled trial, 163 patients post-radical hepatectomy were evaluated for response to one of three antiviral treatments.[44]
- Antiviral treatment significantly decreased hepatocellular carcinoma recurrence with a hazard ratio (HR) of 0.48 (95% confidence interval [CI], 0.32-0.70) and hepatocellular carcinoma-related death with an HR of 0.26 (95% CI, 0.14-0.50), in multivariate Cox analyses.
- Patients who received antiviral treatment had significantly decreased early recurrence (HR, 0.41; 95% CI, 0.27-0.62) and improved liver function 6 months after surgery compared with the controls (P < .001).
Treatment options for progressive or recurrent hepatocellular carcinoma The prognosis for a patient with recurrent or progressive hepatocellular carcinoma is extremely poor.[45] Treatment options for progressive or recurrent hepatocellular carcinoma include the following: - Chemoembolization temporization before transplant or immediate liver transplant, for those with isolated recurrence in the liver.[19,33,34,46]
- Phase I and phase II clinical trials may be appropriate and should be considered.
- Sorafenib has resulted in improved progression-free survival in adults with advanced hepatocellular carcinoma. For adult patients who received sorafenib, the median survival and time to radiologic progression were about 3 months longer than those who received a placebo.[47] A phase II COG trial of single-agent sorafenib has been completed in children and the study results are pending.
References:
-
Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, Md: National Cancer Institute, 2012, Section 29. Also available online. Last accessed May 31, 2017.
-
El-Serag HB, Davila JA, Petersen NJ, et al.: The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139 (10): 817-23, 2003.
-
Chang MH, Chen TH, Hsu HM, et al.: Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res 11 (21): 7953-7, 2005.
-
Cruz O, Laguna A, Vancells M, et al.: Fibrolamellar hepatocellular carcinoma in an infant and literature review. J Pediatr Hematol Oncol 30 (12): 968-71, 2008.
-
Keeffe EB, Pinson CW, Ragsdale J, et al.: Hepatocellular carcinoma in arteriohepatic dysplasia. Am J Gastroenterol 88 (9): 1446-9, 1993.
-
Siciliano M, De Candia E, Ballarin S, et al.: Hepatocellular carcinoma complicating liver cirrhosis in type IIIa glycogen storage disease. J Clin Gastroenterol 31 (1): 80-2, 2000.
-
Ni YH, Chang MH, Hsu HY, et al.: Hepatocellular carcinoma in childhood. Clinical manifestations and prognosis. Cancer 68 (8): 1737-41, 1991.
-
Tsukuma H, Hiyama T, Tanaka S, et al.: Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 328 (25): 1797-801, 1993.
-
González-Peralta RP, Langham MR Jr, Andres JM, et al.: Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 48 (5): 630-5, 2009.
-
Knisely AS, Strautnieks SS, Meier Y, et al.: Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44 (2): 478-86, 2006.
-
Alonso EM, Snover DC, Montag A, et al.: Histologic pathology of the liver in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 18 (2): 128-33, 1994.
-
van Spronsen FJ, Bijleveld CM, van Maldegem BT, et al.: Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2-(2 nitro-4-3 trifluoro- methylbenzoyl)-1, 3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 40 (1): 90-3, 2005.
-
Park WS, Dong SM, Kim SY, et al.: Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res 59 (2): 307-10, 1999.
-
de Laet C, Dionisi-Vici C, Leonard JV, et al.: Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis 8: 8, 2013.
-
De Jesús VR, Adam BW, Mandel D, et al.: Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs. Mol Genet Metab 113 (1-2): 67-75, 2014 Sep-Oct.
-
Bahador A, Dehghani SM, Geramizadeh B, et al.: Liver Transplant for Children With Hepatocellular Carcinoma and Hereditary Tyrosinemia Type 1. Exp Clin Transplant 13 (4): 329-32, 2015.
-
Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 20 (12): 2789-97, 2002.
-
Allan BJ, Wang B, Davis JS, et al.: A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 49 (1): 166-71; discussion 171, 2014.
-
Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006.
-
Eggert T, McGlynn KA, Duffy A, et al.: Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 1 (5): 351-7, 2013.
-
Honeyman JN, Simon EP, Robine N, et al.: Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343 (6174): 1010-4, 2014.
-
Mayo SC, Mavros MN, Nathan H, et al.: Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg 218 (2): 196-205, 2014.
-
Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
-
Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer 97 (8): 2006-12, 2003.
-
Weeda VB, Murawski M, McCabe AJ, et al.: Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma--results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 49 (12): 2698-704, 2013.
-
López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
-
Prokurat A, Kluge P, Kościesza A, et al.: Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta-catenin. Med Pediatr Oncol 39 (5): 510-8, 2002.
-
Exelby PR, Filler RM, Grosfeld JL: Liver tumors in children in the particular reference to hepatoblastoma and hepatocellular carcinoma: American Academy of Pediatrics Surgical Section Survey--1974. J Pediatr Surg 10 (3): 329-37, 1975.
-
Murawski M, Weeda VB, Maibach R, et al.: Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned From the SIOPEL 2 and 3 Studies. J Clin Oncol 34 (10): 1050-6, 2016.
-
Kelly D, Sharif K, Brown RM, et al.: Hepatocellular carcinoma in children. Clin Liver Dis 19 (2): 433-47, 2015.
-
Malek MM, Shah SR, Atri P, et al.: Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery 148 (4): 778-82; discussion 782-4, 2010.
-
Ismail H, Broniszczak D, Kaliciński P, et al.: Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr Transplant 13 (6): 682-92, 2009.
-
Pham TA, Gallo AM, Concepcion W, et al.: Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg 150 (12): 1150-8, 2015.
-
Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
-
Bilik R, Superina R: Transplantation for unresectable liver tumors in children. Transplant Proc 29 (7): 2834-5, 1997.
-
Romano F, Stroppa P, Bravi M, et al.: Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant 15 (6): 573-9, 2011.
-
McAteer JP, Goldin AB, Healey PJ, et al.: Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 17 (8): 744-50, 2013.
-
Zhang Z, Liu Q, He J, et al.: The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer 89 (12): 2606-12, 2000.
-
Yu T, Xu X, Chen B: TACE combined with liver resection versus liver resection alone in the treatment of resectable HCC: a meta-analysis. Chinese-German J Clin Oncol 12 (11): 532-6, 2013.
-
Schmid I, Häberle B, Albert MH, et al.: Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer 58 (4): 539-44, 2012.
-
Zhou XD, Tang ZY: Cryotherapy for primary liver cancer. Semin Surg Oncol 14 (2): 171-4, 1998.
-
Lencioni RA, Allgaier HP, Cioni D, et al.: Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228 (1): 235-40, 2003.
-
Lubienski A: Hepatocellular carcinoma: interventional bridging to liver transplantation. Transplantation 80 (1 Suppl): S113-9, 2005.
-
Yin J, Li N, Han Y, et al.: Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol 31 (29): 3647-55, 2013.
-
Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
-
Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
-
Llovet JM, Ricci S, Mazzaferro V, et al.: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359 (4): 378-90, 2008.
Undifferentiated Embryonal Sarcoma of the LiverIncidence Undifferentiated embryonal sarcoma of the liver (UESL) is a distinct clinical and pathologic entity and accounts for 2% to 15% of pediatric hepatic malignancies.[1] Diagnosis UESL presents as an abdominal mass, often with pain or malaise, usually between the ages of 5 and 10 years. Widespread infiltration throughout the liver and pulmonary metastasis is common. It may appear solid or cystic on imaging, frequently with central necrosis. Distinctive features are characteristic intracellular hyaline globules and marked anaplasia on a mesenchymal background.[2] Many UESL contain diverse elements of mesenchymal cell maturation, such as smooth muscle and fat. Undifferentiated sarcomas, like small cell undifferentiated hepatoblastomas, should be examined for loss of INI1 expression by immunohistochemistry to help rule out rhabdoid tumor of the liver. It is important to make the diagnostic distinction between UESL and biliary tract rhabdomyosarcoma because they share some common clinical and pathologic features but treatment differs between the two, as shown in Table 6.[1] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.) Table 6. Diagnostic Differences Between Undifferentiated Embryonal Sarcoma of the Liver and Biliary Tract Rhabdomyosarcomaa | Undifferentiated Embryonal Sarcoma of the Liver | Biliary Tract Rhabdomyosarcoma |
|---|
| a Adapted from Nicol et al.[1] | | Age at Diagnosis | Median age 10.5 y | Median age 3.4 y | | Tumor Location | Often arises in the right lobe of the liver | Often arises in the hilum of the liver | | Biliary Obstruction | Unusual | Frequent; jaundice is a common presenting symptom | | Treatment | Surgery and chemotherapy | Surgery (usually biopsy only), radiation therapy, and chemotherapy | Histology Distinctive histologic features are intracellular hyaline globules and marked anaplasia on a mesenchymal background.[2] Strong clinical and histological evidence suggests that UESL can arise within preexisting mesenchymal hamartomas of the liver, which are large benign multicystic masses that present in the first 2 years of life.[1] In a report of 11 cases of UESL, five arose in association with mesenchymal hamartomas of the liver, and transition zones between the histologies were noted.[3] Many mesenchymal hamartomas of the liver have a characteristic translocation with a breakpoint at 19q13.4 and several UESLs have the same translocation.[4,5] Some UESLs arising from mesenchymal hamartomas of the liver may have complex karyotypes not involving 19q13.4.[4] Treatment Options for Undifferentiated Embryonal Sarcoma of the Liver UESL is rare. Only small series have been published regarding treatment. The overall survival (OS) of children with UESL appears to be substantially better than 50% when combining reports, although all series are small and most may be selected to report successful treatment.[6]; [7][Level of evidence: 3iiA]; [8,9,10,11,12,13,14,15,16,17][Level of evidence: 3iiiA] The Childhood Cancer Database, which does not provide central review of pathology or reliable details of nonsurgical treatment, reported on 103 children with UESL diagnosed between 1998 and 2012. The 5-year OS was 86% for all patients and 92% for those treated with combination surgery and chemotherapy. A multivariate analysis of the nonsurgical data revealed statistically significant poorer outcomes for patients with tumors larger than 15 cm. Seven of ten children who presented with metastases and ten of ten children who received orthotopic liver transplantation survived at least 5 years, but details of their treatment are not presented.[18] Treatment options for UESL include the following: - Surgical resection and chemotherapy.
- Liver transplantation, for unresectable tumors.
The generally accepted approach is resection of the primary tumor mass in the liver when possible.[18] Use of aggressive chemotherapy regimens seems to have improved the OS. Neoadjuvant chemotherapy can be effective in decreasing the size of an unresectable primary tumor mass, resulting in resectability.[8,9,10,11] Most patients are treated with chemotherapy regimens often used for pediatric rhabdomyosarcoma or Ewing sarcoma without cisplatin.[6]; [7,19][Level of evidence: 3iiA]; [8,9,10,11,12,13,14,15,16][Level of evidence: 3iiiA] Evidence (surgical resection and chemotherapy): - In the only prospective series treating UESL, which came from the Italian and German Soft Tissue Sarcoma Cooperative Groups, patients were treated with (1) conservative surgery or (2) biopsy followed by neoadjuvant chemotherapy consisting of varying combinations of vincristine, cyclophosphamide, dactinomycin, doxorubicin, and ifosfamide. Disease evaluation, usually after four cycles of chemotherapy, was followed by second-look surgery when appropriate to try to remove residual primary tumor followed by additional and/or adjuvant chemotherapy.[12]
- Ten of 17 patients survived in their first complete remission, and one patient survived in third complete remission.
- In a single-center retrospective report, five patients with UESL were treated with surgery and adjuvant chemotherapy consisting of vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide. Four patients were stage I and one patient was stage II. One patient received abdominal radiation for tumor rupture.[17][Level of evidence: 3iiiA]
- All patients are alive (range, 5-19 years), with 100% event-free survival and OS.
Liver transplantation has on occasion been used successfully to treat an otherwise unresectable primary tumor.[14,16,18,20] Treatment options under clinical evaluation The following is an example of a national and/or institutional clinical trial that is currently being conducted. Information about ongoing clinical trials is available from the NCI website. - ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation or chemotherapy (ifosfamide/etoposide) and radiation in pediatric and adult patients newly diagnosed with unresected, intermediate-risk and high-risk nonrhabdomyosarcomatous STS. Subsequently, this trial will compare the rates of near complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation versus preoperative chemoradiation alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive (i.e., histologies of undifferentiated sarcoma, synovial sarcoma, and embryonal sarcoma of the liver) adult and pediatric nonrhabdomyosarcomatous STS; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous STS.
References:
-
Nicol K, Savell V, Moore J, et al.: Distinguishing undifferentiated embryonal sarcoma of the liver from biliary tract rhabdomyosarcoma: a Children's Oncology Group study. Pediatr Dev Pathol 10 (2): 89-97, 2007 Mar-Apr.
-
Stocker JT: Hepatic tumors in children. Clin Liver Dis 5 (1): 259-81, viii-ix, 2001.
-
Shehata BM, Gupta NA, Katzenstein HM, et al.: Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: a review of eleven cases. Pediatr Dev Pathol 14 (2): 111-6, 2011 Mar-Apr.
-
Stringer MD, Alizai NK: Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg 40 (11): 1681-90, 2005.
-
O'Sullivan MJ, Swanson PE, Knoll J, et al.: Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol 4 (5): 482-9, 2001 Sep-Oct.
-
Walther A, Geller J, Coots A, et al.: Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl 20 (2): 191-9, 2014.
-
Ismail H, Dembowska-Bagińska B, Broniszczak D, et al.: Treatment of undifferentiated embryonal sarcoma of the liver in children--single center experience. J Pediatr Surg 48 (11): 2202-6, 2013.
-
Chowdhary SK, Trehan A, Das A, et al.: Undifferentiated embryonal sarcoma in children: beware of the solitary liver cyst. J Pediatr Surg 39 (1): E9-12, 2004.
-
Baron PW, Majlessipour F, Bedros AA, et al.: Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg 11 (1): 73-5, 2007.
-
Kim DY, Kim KH, Jung SE, et al.: Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg 37 (10): 1419-23, 2002.
-
Webber EM, Morrison KB, Pritchard SL, et al.: Undifferentiated embryonal sarcoma of the liver: results of clinical management in one center. J Pediatr Surg 34 (11): 1641-4, 1999.
-
Bisogno G, Pilz T, Perilongo G, et al.: Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer 94 (1): 252-7, 2002.
-
Urban CE, Mache CJ, Schwinger W, et al.: Undifferentiated (embryonal) sarcoma of the liver in childhood. Successful combined-modality therapy in four patients. Cancer 72 (8): 2511-6, 1993.
-
Okajima H, Ohya Y, Lee KJ, et al.: Management of undifferentiated sarcoma of the liver including living donor liver transplantation as a backup procedure. J Pediatr Surg 44 (2): e33-8, 2009.
-
Weitz J, Klimstra DS, Cymes K, et al.: Management of primary liver sarcomas. Cancer 109 (7): 1391-6, 2007.
-
Plant AS, Busuttil RW, Rana A, et al.: A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol 35 (6): 451-5, 2013.
-
Mathias MD, Ambati SR, Chou AJ, et al.: A single-center experience with undifferentiated embryonal sarcoma of the liver. Pediatr Blood Cancer 63 (12): 2246-2248, 2016.
-
Shi Y, Rojas Y, Zhang W, et al.: Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the National Cancer Database. Pediatr Blood Cancer 64 (4): , 2017.
-
Merli L, Mussini C, Gabor F, et al.: Pitfalls in the surgical management of undifferentiated sarcoma of the liver and benefits of preoperative chemotherapy. Eur J Pediatr Surg 25 (1): 132-7, 2015.
-
Kelly MJ, Martin L, Alonso M, et al.: Liver transplant for relapsed undifferentiated embryonal sarcoma in a young child. J Pediatr Surg 44 (12): e1-3, 2009.
Infantile Choriocarcinoma of the LiverChoriocarcinoma of the liver is a very rare tumor that appears to originate in the placenta during gestation and presents with a liver mass in the first few months of life. Metastasis from the placenta to maternal tissues occurs in many cases, necessitating beta-human chorionic gonadotropin (beta-hCG) testing of the mother. Infants are often unstable at diagnosis because of hemorrhage of the tumor. Clinical diagnosis may be made without biopsy based on tumor imaging of the liver associated with extremely high serum beta-hCG levels and normal alpha-fetoprotein (AFP) levels for age.[1] Cytotrophoblasts and syncytiotrophoblasts are both present. The former are closely packed nests of medium-sized cells with clear cytoplasm, distinct cell margins, and vesicular nuclei. The latter are very large multinucleated syncytia formed from the cytotrophoblasts.[2] Treatment Options for Infantile Choriocarcinoma of the Liver Treatment options for infantile choriocarcinoma of the liver include the following: - Surgical resection.[1]
- Chemotherapy followed by surgical resection.
Initial surgical removal of the tumor mass may be difficult because of its friability and hemorrhagic tendency. Often surgical removal of the primary tumor is performed after neoadjuvant chemotherapy.[1] Maternal gestational trophoblastic tumors are exquisitely sensitive to methotrexate, and many women, including those with distant metastases, are cured with single-agent chemotherapy. Maternal and infantile choriocarcinoma both come from the same placental malignancy. The combination of cisplatin, etoposide, and bleomycin, as used in other pediatric germ cell tumors, has been effective in some patients and is followed by resection of residual mass. Use of neoadjuvant methotrexate in infantile choriocarcinoma, although often resulting in a response, has not been uniformly successful.[1] References:
-
Yoon JM, Burns RC, Malogolowkin MH, et al.: Treatment of infantile choriocarcinoma of the liver. Pediatr Blood Cancer 49 (1): 99-102, 2007.
-
Olson T, Schneider D, Perlman E: Germ cell tumors. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 6th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2011, pp 1045-1067.
Current Clinical TrialsCheck the list of NCI-supported cancer clinical trials that are now accepting patients with childhood liver cancer. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. Changes to This Summary (04 / 14 / 2017)The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Hepatoblastoma Added text to state that a multivariate analysis of prognostic factors has been published to help develop a new risk group classification for hepatoblastoma; this classification was used to generate a risk stratification schema to be used in international clinical trials (cited Meyers et al. as reference 37). Added text about the results of a Children's Oncology Group study that included 30 children with metastatic hepatoblastoma who were treated with vincristine and irinotecan before standard cisplatin-based therapy (cited Katzenstein et al. as reference 85). Added text to state that percutaneous ablation techniques may also be considered as treatment options for recurrent or progressive hepatoblastoma (cited Liu et al. as reference 95). Hepatocellular Carcinoma Added Nonviral liver injury as a new subsection. Undifferentiated Embryonal Sarcoma of the Liver (UESL) Added text about a report from the Childhood Cancer Database that included 103 children with UESL diagnosed between 1998 and 2012 (cited Shi et al. as reference 18). This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages. About This PDQ SummaryPurpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood liver cancer. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: - be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Childhood Liver Cancer Treatment are: - Denise Adams, MD (Children's Hospital Boston)
- Christopher N. Frantz, MD (Alfred I. duPont Hospital for Children)
- Andrea A. Hayes-Jordan, MD, FACS, FAAP (M.D. Anderson Cancer Center)
- Karen J. Marcus, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Thomas A. Olson, MD (AFLAC Cancer Center and Blood Disorders Service of Children's Healthcare of Atlanta - Egleston Campus)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Liver Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/liver/hp/child-liver-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389232] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2017-04-14 Last modified on: 8 September 2017
|
|