Childhood Central Nervous System Embryonal Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]
Childhood Central Nervous System Embryonal Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]Skip to the navigationGeneral Information About Central Nervous System (CNS) Embryonal Tumors The PDQ childhood brain tumor treatment summaries are organized primarily according to the World Health Organization (WHO) classification of nervous system tumors.[1] For a full description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, refer to the PDQ summary on Childhood Brain and Spinal Cord Tumors Treatment Overview. Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[2] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.) Primary brain tumors are a diverse group of diseases that together constitute the most common solid tumor of childhood. Brain tumors are classified according to histology, but tumor location and extent of spread are important factors that affect treatment and prognosis. Immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Disease Overview Embryonal tumors are a collection of biologically heterogeneous lesions that share the tendency to disseminate throughout the nervous system via cerebrospinal fluid (CSF) pathways. Although there is significant variability, histologically these tumors are grouped together because they are at least partially composed of hyperchromatic cells (blue cell tumors on standard staining) with little cytoplasm, which are densely packed and demonstrate a high degree of mitotic activity. Other histologic and immunohistochemical features, such as the degree of apparent cellular transformation along identifiable cell lineages (ependymal, glial, etc.), can be used to separate these tumors to some degree. However, a convention, which has been accepted by the WHO, also separates these tumors on the basis of presumed location of origin within the central nervous system (CNS). Molecular studies have substantiated the differences between tumors arising in different areas of the brain and give partial credence to this classification approach.[3] As of 2016, the WHO has proposed an integrated phenotypic and genotypic classification system for CNS tumors.[1] The term primitive neuroectodermal tumor (PNET) has been removed from the newest WHO diagnostic lexicon, although some rare entities (e.g., medulloepithelioma) have remained. A molecularly distinct entity, embryonal tumor with multilayered rosettes (ETMR), C19MC-altered, has been added. The WHO classification will be updated as other molecularly distinct entities are defined. The pathologic diagnosis of embryonal tumors is based primarily on histological and immunohistological microscopic features. However, molecular genetic studies are employed increasingly to subclassify embryonal tumors. These molecular genetic findings are also being utilized for risk stratification and treatment planning.[4,5,6,7] The most recent WHO categorization of embryonal tumors is as follows:[1] - Medulloblastoma, genetically defined.
- Medulloblastoma, WNT-activated.
- Medulloblastoma, SHH-activated and TP53-mutant.
- Medulloblastoma, SHH-activated and TP53-wildtype.
- Medulloblastoma, non-WNT/non-SHH.
- Medulloblastoma, group 3.
- Medulloblastoma, group 4.
- Medulloblastoma, histologically defined.
- Medulloblastoma, classic.
- Medulloblastoma, desmoplastic/nodular.
- Medulloblastoma with extensive nodularity.
- Medulloblastoma, large cell/anaplastic.
- Medulloblastoma, not otherwise specified (NOS).
- ETMR, C19MC-altered.
- ETMR, NOS.
- Medulloepithelioma.
- CNS neuroblastoma.
- CNS ganglioneuroblastoma.
- CNS embryonal tumor, NOS.
- CNS atypical teratoid/rhabdoid tumor. (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment for more information about CNS atypical teratoid/rhabdoid tumors.)
- CNS embryonal tumor with rhabdoid features.
Pineoblastoma, which in the past was conventionally grouped with embryonal tumors, is categorized by the WHO as a pineal parenchymal tumor. Given that therapies for pineoblastomas are quite similar to those utilized for embryonal tumors, pineoblastomas are discussed in this summary. A somewhat closely aligned tumor, pineal parenchymal tumor of intermediate differentiation, has recently been identified but is not considered an embryonal tumor and primarily arises in adults.[1] The prognosis for embryonal tumors and pineoblastomas varies greatly depending on the following:[1,8] - Extent of CNS disease at the time of diagnosis.
- Age at diagnosis.
- Amount of residual disease after definitive surgery.
- Tumor histopathology.
- Biological/molecular tumor cell characteristics.
It has become increasingly clear, especially for medulloblastomas, that outcome is also related to the molecular characteristics of the tumor, but this has not been definitively shown for other embryonal tumors.[3,6,7,9,10,11] Overall survival rates range from 40% to 90%, depending on the molecular subtype of the medulloblastoma and possibly other factors, such as extent of dissemination at time of diagnosis and degree of resection. Children who survive for 5 years are considered cured of their tumor. Survival rates for other embryonal tumors are generally poorer, ranging from less than 5% to 50%; specifics are discussed within each subgroup in the summary.[12,13,14,15]
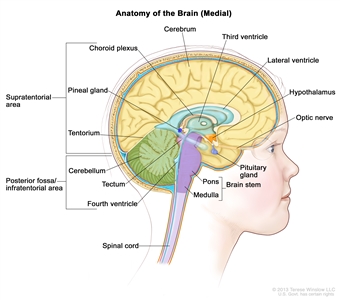
Figure 1. Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The posterior fossa is the region below the tentorium, which separates the cortex from the cerebellum and essentially denotes the region containing the brain stem, cerebellum, and fourth ventricle.
Incidence Embryonal tumors comprise 20% to 25% of primary CNS tumors (malignant brain tumors and pilocytic astrocytomas) arising in children. These tumors occur along the pediatric age spectrum but tend to cluster early in life. The incidence of embryonal tumors in children aged 1 to 9 years is fivefold to tenfold higher than is the incidence of embryonal tumors in adults.[16,17] Table 1. Annual Incidence Rates for Childhood Central Nervous System Embryonal Tumors According to Age| Age Group (y) | Annual Incidence Rate (Cases per 1 Million) |
|---|
| <5 | 11 | | 5-9 | 7 | | 10-19 | 3-4 | Medulloblastomas comprise the vast majority of pediatric embryonal tumors and by definition arise in the posterior fossa (refer to Figure 1), where they constitute approximately 40% of all posterior fossa tumors. Other forms of embryonal tumors each make up 2% or less of all childhood brain tumors. Clinical Features The clinical features of childhood embryonal tumors depend on the location of the tumor and the age of the child at the time of presentation. Embryonal tumors tend to be fast-growing tumors and are usually diagnosed within 3 months of initial onset of symptoms.[18] Medulloblastoma In approximately 80% of children, medulloblastomas arise in the region of the fourth ventricle. Most of the early symptomatology is related to blockage of CSF and resultant accumulation of CSF in the brain, termed hydrocephalus. Children with medulloblastoma are usually diagnosed within 2 to 3 months of onset of symptoms and commonly present with the following:[19] - Relatively abrupt onset of headaches, especially in the morning on waking.
- Nausea and/or vomiting.
- Lethargy.
- Ataxia, including truncal unsteadiness.
- Some degree of nystagmus.
- Papilledema.
Twenty percent of patients with medulloblastoma will not have hydrocephalus at the time of diagnosis and are more likely to present initially with cerebellar deficits. For example, more laterally positioned medulloblastomas of the cerebellum may not result in hydrocephalus and, because of their location, are more likely to result in lateralizing cerebellar dysfunction (appendicular ataxia) manifested by unilateral dysmetria, unsteadiness, and weakness of the sixth and seventh nerves on the same side as the tumor. Later, as the tumor grows toward the midline and blocks CSF, the more classical symptoms associated with hydrocephalus become evident. Cranial nerve findings are less common, except for unilateral or bilateral sixth nerve palsies, which are usually related to hydrocephalus.[19] At times, medulloblastomas will present explosively, with the acute onset of lethargy and unconsciousness due to hemorrhage within the tumor. In infants, the presentation of medulloblastoma is more variable and may include the following: - Nonspecific lethargy.
- Psychomotor delays.
- Loss of developmental milestones.
- Feeding difficulties.
On examination, there may be bulging of the anterior fontanel due to increased intracranial pressure and abnormal eye movements, including eyes that are deviated downward (the so-called sun setting sign) due to loss of upgaze secondary to compression of the tectum of the midbrain. Hereditary cancer predisposition syndromes associated with medulloblastoma Up to 10% of medulloblastoma cases arise in the setting of hereditary cancer predisposition syndromes.[20] Syndromes known to be associated with medulloblastoma include the following: - Turcot syndrome (related to germline mutations in APC).[21]
- Rubinstein-Taybi syndrome (related to germline mutations in CREBBP).[22,23,24]
- Gorlin syndrome (also known as basal cell nevus syndrome or nevoid basal cell carcinoma syndrome, associated with germline PTCH1 and SUFU mutations).[25,26,27,28,29] The risk of developing medulloblastoma in patients with Gorlin syndrome appears to be higher in those with SUFU mutations than in those with PTCH1 germline mutations. In one study, 2 of 115 individuals (1.7%) with Gorlin syndrome and a PTCH1 mutation developed a pathology-proven medulloblastoma, compared with three of nine individuals (33%) from three families with SUFU-related Gorlin syndrome. Each of the SUFU-related patients developed medulloblastoma before age 3 years.[29]
- Li-Fraumeni syndrome (related to germline mutations in TP53).[30,31]
- Fanconi anemia.[32,33]
Sometimes medulloblastoma may be the initial manifestation of the presence of germline mutations in these predisposition genes. Nonmedulloblastoma embryonal tumors For nonmedulloblastoma embryonal tumors, presentation is also relatively rapid and depends on the location of the tumor in the nervous system. Pineoblastomas often result in hydrocephalus due to blockage of CSF at the third ventricular level and other symptoms related to pressure on the back of the brain stem in the tectal region. Symptoms may include a constellation of abnormalities in eye movements manifested by pupils that react poorly to light but better to accommodation, loss of upgaze, retraction or convergence nystagmus, and lid retraction (Parinaud syndrome). As they grow, these tumors may also cause hemiparesis and ataxia. Supratentorial embryonal tumors (refer to Figure 1) will result in focal neurologic deficits, such as hemiparesis and visual field loss, depending on which portion of the cerebral cortex is involved. They may also result in seizures and obtundation. Nonmedulloblastoma embryonal tumors may occur anywhere in the CNS, and presentation is variable. Usually there is significant neurologic dysfunction associated with lethargy and vomiting. Pineoblastoma is associated with germline mutations in the retinoblastoma (RB1) gene, with the term trilateral retinoblastoma used to refer to ocular retinoblastoma in combination with a histologically similar brain tumor generally arising in the pineal gland or other midline structures. Historically, intracranial tumors have been reported in 5% to 15% of children with heritable retinoblastoma.[34] Rates of pineoblastoma among children with heritable retinoblastoma who undergo current treatment programs may be lower than these historical estimates.[35,36,37] Baseline brain imaging of children with retinoblastoma may identify pineoblastoma at an early stage and increase the likelihood of successful treatment.[38,39] Germline DICER1 mutations have also been reported in patients with pineoblastoma.[40] Among 18 patients with pineoblastoma, three patients with DICER1 germline mutations were identified, and an additional three patients known to be carriers of germline DICER1 mutations developed pineoblastoma. The DICER1 mutations in patients with pineoblastoma appear to be distinct from the mutations observed in DICER1 syndrome-related tumors such as pleuropulmonary blastoma.[40] Diagnostic and Staging Evaluation Diagnosis is usually readily made by either magnetic resonance imaging (MRI) or computed tomography (CT) scan. MRI is preferable because the anatomic relationship between the tumor and surrounding brain and tumor dissemination is better visualized with this method.[18] After diagnosis, evaluation of embryonal tumors is quite similar, essentially independent of the histologic subtype and the location of the tumor. Given the tendency of these tumors to disseminate throughout the CNS early in the course of illness, imaging evaluation of the neuraxis by means of MRI of the entire brain and spine is indicated. Preferably this is done before surgery, to avoid postoperative artifacts, especially blood. Such imaging can be difficult to interpret and must be performed in at least two planes, with and without the use of contrast enhancement (gadolinium).[41] After surgery, imaging of the primary tumor site is indicated to determine the extent of residual disease. In addition, lumbar CSF analysis is performed, if deemed safe. Neuroimaging and CSF evaluation are considered complementary because as many as 10% of patients will have evidence of free-floating tumor cells in the CSF without clear evidence of leptomeningeal disease on MRI scan.[42] CSF analysis is conventionally done 10 to 21 days after surgery. If CSF is obtained within 10 days of the operation, detection of tumor cells within the spinal fluid is possibly related to the surgical procedure. In most staging systems, if fluid is obtained in the first few days after surgery and found to be positive, the positivity must be confirmed by a subsequent spinal tap to be considered diagnostically significant. When obtainment of fluid by lumbar spinal tap is deemed unsafe, ventricular fluid can be obtained; however, it may not be as sensitive as lumbar fluid assessment.[42] Because embryonal tumors are very rarely metastatic to the bone, bone marrow, or other body sites at the time of diagnosis, studies such as bone marrow aspirates, chest x-rays, or bone scans are not indicated, unless there are symptoms or signs suggesting organ involvement. Additional diagnostic studies for patients with desmoplastic medulloblastoma Patients with desmoplastic tumors with extensive nodularity should be carefully evaluated for stigmata of Gorlin syndrome.[25] One report observed that medulloblastoma with extensive nodularity (MBEN) was associated with Gorlin syndrome in 5 of 12 cases.[25] Gorlin syndrome, also called nevoid basal cell carcinoma syndrome, is an autosomal dominant disorder in which those affected are predisposed to the development of basal cell carcinomas later in life, especially in skin in the radiation portal. The syndrome can be diagnosed early in life by detection of characteristic dermatological and skeletal features such as keratocysts of the jaw, bifid or fused ribs, macrocephaly, and calcifications of the falx.[25] Prognostic Factors Various clinical and biologic parameters have been shown to be associated with the likelihood of disease control of embryonal tumors after treatment.[5] The significance of many of these factors have been shown to be predictive for medulloblastomas, although some are used to assign risk, to some degree, for other embryonal tumors. Parameters that are most frequently utilized to predict outcome include the following:[43,44] - Extent of CNS disease at diagnosis.
- Age at diagnosis.
- Amount of residual disease after definitive surgery.
- Tumor histopathology.
- Biological/molecular tumor cell characteristics.
In older studies, the presence of brain stem involvement in children with medulloblastoma was found to be a prognostic factor; it has not been found to be of predictive value in subsequent studies utilizing both radiation and chemotherapy.[41,43] Extent of CNS disease at diagnosis Patients with disseminated CNS disease at diagnosis are at highest risk of disease relapse.[42,43,44] Ten percent to 40% of patients with medulloblastoma have CNS dissemination at diagnosis, with infants having the highest incidence and adolescents and adults having the lowest incidence. Nonmedulloblastoma embryonal tumors and pineoblastomas may also be disseminated at the time of diagnosis, although the incidence of dissemination may be somewhat less than that of medulloblastomas, with dissemination at diagnosis being documented in approximately 10% to 20% of patients.[12,13] Patients with nonmedulloblastoma embryonal tumors and pineoblastomas who have disseminated disease at the time of diagnosis have a poor overall survival, with reported survival rates at 5 years ranging from 10% to 30%.[12,13,14,15] Age at diagnosis Age younger than 3 years at diagnosis (except for desmoplastic medulloblastoma/medulloblastoma with extensive nodularity) portends an unfavorable outcome for those with medulloblastoma and, possibly, other embryonal tumors.[45,46,47,48,49] Amount of residual disease after definitive surgery Extent of resection determined during surgery has been supplanted by postoperative MRI measurement of the amount of residual disease after definitive surgery as a predictor of outcome.[41] In older studies, the extent of resection for medulloblastomas was found to be related to survival.[43,44,50,51] A HIrnTumor and International Society of Paediatric Oncology (HIT-SIOP) study of 340 children reported that residual disease (>1.5 cm2) connoted a poorer 5-year event-free survival.[52] Extent of resection after surgery is still used to separate patients into risk groups, with patients having more than 1.5 cm2 residual disease stratified into high-risk groups. An international, retrospective, collaborative study included 787 medulloblastoma patients of all ages who were treated in a variety of ways and incorporated molecular subgrouping and clinical factors in the analysis. The multivariate analysis found that subtotal resection, but not near-total resection (<1.5 cm2 tumor remaining), was associated with inferior progression-free survival compared with gross-total resection. This study suggests that attempts to completely remove the tumor, especially when the likelihood of neurological morbidity is high, are not warranted, as there appears to be little or no benefit to gross-total resection, when compared with near-total resection. It gives some credence to the present approach where patients with more than 1.5 cm2 of disease are considered higher-risk patients. Prospective studies are needed to better define the impact of extent of resection on outcome within molecularly defined subgroups.[53] In patients with other forms of embryonal tumors, the extent of resection has not been definitively shown to impact survival.[14] However, in a Children's Oncology Group (COG) study of 66 children with supratentorial embryonal tumors, extent of resection was found to be prognostic for those with localized disease at the time of diagnosis.[54] Tumor histopathology For medulloblastomas, histopathologic features such as large cell variant, anaplasia, and desmoplasia have been shown in retrospective analyses to correlate with outcome.[46,55,56] In prospective studies, immunohistochemical and histopathologic findings have not predicted outcome in children older than 3 years at diagnosis, with the exception of the anaplasia/large cell variant, which has been associated with poorer prognosis.[11,41,57] Several studies have observed that the histologic finding of desmoplasia, seen in patients aged 3 years and younger with desmoplastic medulloblastoma, especially MBEN, connotes a significantly better prognosis compared with outcome for infants and young children with classic or large cell/anaplastic medulloblastoma.[11,25,45,46,47]; [48][Level of evidence: 2A] For other embryonal tumors, histologic variations have not been associated with differing outcome. Biological/molecular tumor cell characteristics Genomic analyses (including RNA gene expression and DNA methylation profiles, as well as DNA sequencing to identify mutations) on both fresh-frozen and formalin-fixed, paraffin-embedded sections have identified molecular subtypes of medulloblastoma.[4,5,6,7,9,10,58,59,60,61,62,63,64,65] These subtypes include those characterized by WNT pathway activation and sonic hedgehog (SHH) pathway activation, as well as additional subgroups characterized by MYC or MYCN alterations and other genomic alterations.[4,5,6,7,9,10,58,59,60,61,62,63,64] Patients whose tumors show WNT pathway activation usually have an excellent prognosis, while patients with SHH pathway-activated tumors generally show an intermediate prognosis. Outcome for the remaining patients is less favorable than that for patients with WNT pathway activation. Mutations in medulloblastoma cases are observed in a subtype-specific manner, with CTNNB1 mutations observed in the WNT subtype and with PTCH1, SMO, and SUFU mutations observed in the SHH subtype. The prognostic significance of recurring mutations is closely aligned with that of the molecular subtype with which they are associated.[5,66] At recurrence, the subtype remains unchanged from the original molecular subtype at diagnosis.[67] Refer to the Biologically/molecularly defined subtypes of medulloblastoma section of this summary for more information about the subtypes of medulloblastoma. For nonmedulloblastoma embryonal tumors, integrative genomic analysis has also identified molecular subtypes with different outcomes. (Refer to the Cellular and Molecular Classification of CNS Embryonal Tumors section of this summary for more detailed information.) Follow-up After Treatment Relapse in children with embryonal tumors is most likely to occur within the first 18 months of diagnosis.[52,68] Surveillance imaging of the brain and spine is usually undertaken at routine intervals during and after treatment (refer to Table 2). The frequency of such imaging, designed to detect recurrent disease at an early, asymptomatic state, has been arbitrarily determined and has not been shown to clearly influence survival.[69,70,71] Growth hormone replacement therapy has not been shown to increase the likelihood of disease relapse.[47] Table 2. Surveillance Testing During and After Treatment for Medulloblastoma and Other Central Nervous System Embryonal Tumors| Surveillance Period | Frequency of Visits During Surveillance Period | Testing |
|---|
| MRI = magnetic resonance imaging. | | a For pineoblastoma, continue spinal evaluations every 6 months until 5 years from diagnosis. Although these suggestions are based on a small sample size, there is evidence to continue surveillance testing of the spine until 5 years after diagnosis.[72] | | First 3 years after diagnosis | Every 3 months | Physical exam | | Imaging of the brain every 3 months for the first 3 years, then every 6 months for the ensuing 2 years, and then as per preference of the treating physician or per protocol; MRI of the spine every 3 months for the first 2 years, then every 6 months for 1 year, and then as per preference of the treating physician or per protocol.a | | Endocrinology evaluation once a year | | Neuropsychologic testing every 1-2 years | | 3-5 years after diagnosis | Every 6 months | Physical exam | | Imaging of the brain and spine once a year | | Endocrinology evaluation once a year | | Neuropsychologic testing every 1-2 years | | More than 5 years after diagnosis | Once a year | Physical exam | | Imaging of the brain once a year | | Endocrinology evaluation once a year | | Neuropsychologic testing every 1-2 years (optional) | References:
-
Louis DN, Perry A, Reifenberger G, et al.: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6): 803-20, 2016.
-
Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
-
Pomeroy SL, Tamayo P, Gaasenbeek M, et al.: Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415 (6870): 436-42, 2002.
-
Pfister S, Remke M, Benner A, et al.: Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol 27 (10): 1627-36, 2009.
-
Taylor MD, Northcott PA, Korshunov A, et al.: Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123 (4): 465-72, 2012.
-
Kool M, Koster J, Bunt J, et al.: Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3 (8): e3088, 2008.
-
Ellison DW, Onilude OE, Lindsey JC, et al.: beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol 23 (31): 7951-7, 2005.
-
Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010.
-
Thompson MC, Fuller C, Hogg TL, et al.: Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24 (12): 1924-31, 2006.
-
Northcott PA, Korshunov A, Witt H, et al.: Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29 (11): 1408-14, 2011.
-
Rutkowski S, von Hoff K, Emser A, et al.: Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 28 (33): 4961-8, 2010.
-
Cohen BH, Zeltzer PM, Boyett JM, et al.: Prognostic factors and treatment results for supratentorial primitive neuroectodermal tumors in children using radiation and chemotherapy: a Childrens Cancer Group randomized trial. J Clin Oncol 13 (7): 1687-96, 1995.
-
Reddy AT, Janss AJ, Phillips PC, et al.: Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer 88 (9): 2189-93, 2000.
-
Timmermann B, Kortmann RD, Kühl J, et al.: Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol 20 (3): 842-9, 2002.
-
Jakacki RI, Zeltzer PM, Boyett JM, et al.: Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol 13 (6): 1377-83, 1995.
-
Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, Md: National Cancer Institute, 2012, Section 29. Also available online. Last accessed May 31, 2017.
-
Smoll NR, Drummond KJ: The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 19 (11): 1541-4, 2012.
-
Chintagumpala MM, Paulino A, Panigrahy A, et al.: Embryonal and pineal region tumors. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 671-99.
-
Ramaswamy V, Remke M, Shih D, et al.: Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer 61 (7): 1190-4, 2014.
-
Zhang J, Walsh MF, Wu G, et al.: Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med 373 (24): 2336-46, 2015.
-
Hamilton SR, Liu B, Parsons RE, et al.: The molecular basis of Turcot's syndrome. N Engl J Med 332 (13): 839-47, 1995.
-
Taylor MD, Mainprize TG, Rutka JT, et al.: Medulloblastoma in a child with Rubenstein-Taybi Syndrome: case report and review of the literature. Pediatr Neurosurg 35 (5): 235-8, 2001.
-
Miller RW, Rubinstein JH: Tumors in Rubinstein-Taybi syndrome. Am J Med Genet 56 (1): 112-5, 1995.
-
Bourdeaut F, Miquel C, Richer W, et al.: Rubinstein-Taybi syndrome predisposing to non-WNT, non-SHH, group 3 medulloblastoma. Pediatr Blood Cancer 61 (2): 383-6, 2014.
-
Garrè ML, Cama A, Bagnasco F, et al.: Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome--a new clinical perspective. Clin Cancer Res 15 (7): 2463-71, 2009.
-
Pastorino L, Ghiorzo P, Nasti S, et al.: Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A 149A (7): 1539-43, 2009.
-
Brugières L, Remenieras A, Pierron G, et al.: High frequency of germline SUFU mutations in children with desmoplastic/nodular medulloblastoma younger than 3 years of age. J Clin Oncol 30 (17): 2087-93, 2012.
-
Evans DG, Farndon PA, Burnell LD, et al.: The incidence of Gorlin syndrome in 173 consecutive cases of medulloblastoma. Br J Cancer 64 (5): 959-61, 1991.
-
Smith MJ, Beetz C, Williams SG, et al.: Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol 32 (36): 4155-61, 2014.
-
Li FP, Fraumeni JF Jr: Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst 43 (6): 1365-73, 1969.
-
Pearson AD, Craft AW, Ratcliffe JM, et al.: Two families with the Li-Fraumeni cancer family syndrome. J Med Genet 19 (5): 362-5, 1982.
-
de Chadarévian JP, Vekemans M, Bernstein M: Fanconi's anemia, medulloblastoma, Wilms' tumor, horseshoe kidney, and gonadal dysgenesis. Arch Pathol Lab Med 109 (4): 367-9, 1985.
-
Offit K, Levran O, Mullaney B, et al.: Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst 95 (20): 1548-51, 2003.
-
Kivelä T: Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 17 (6): 1829-37, 1999.
-
Abramson DH, Dunkel IJ, Marr BP, et al.: Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol 156 (6): 1319-20, 2013.
-
Turaka K, Shields CL, Meadows AT, et al.: Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer 59 (1): 121-5, 2012.
-
Ramasubramanian A, Kytasty C, Meadows AT, et al.: Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol 156 (4): 825-9, 2013.
-
Rodjan F, de Graaf P, Brisse HJ, et al.: Trilateral retinoblastoma: neuroimaging characteristics and value of routine brain screening on admission. J Neurooncol 109 (3): 535-44, 2012.
-
de Graaf P, Göricke S, Rodjan F, et al.: Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol 42 (1): 2-14, 2012.
-
de Kock L, Sabbaghian N, Druker H, et al.: Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol 128 (4): 583-95, 2014.
-
Packer RJ, Gajjar A, Vezina G, et al.: Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24 (25): 4202-8, 2006.
-
Fouladi M, Gajjar A, Boyett JM, et al.: Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 17 (10): 3234-7, 1999.
-
Zeltzer PM, Boyett JM, Finlay JL, et al.: Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol 17 (3): 832-45, 1999.
-
Yao MS, Mehta MP, Boyett JM, et al.: The effect of M-stage on patterns of failure in posterior fossa primitive neuroectodermal tumors treated on CCG-921: a phase III study in a high-risk patient population. Int J Radiat Oncol Biol Phys 38 (3): 469-76, 1997.
-
Leary SE, Zhou T, Holmes E, et al.: Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children's oncology group. Cancer 117 (14): 3262-7, 2011.
-
Giangaspero F, Perilongo G, Fondelli MP, et al.: Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg 91 (6): 971-7, 1999.
-
von Bueren AO, von Hoff K, Pietsch T, et al.: Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 13 (6): 669-79, 2011.
-
Rutkowski S, Gerber NU, von Hoff K, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol 11 (2): 201-10, 2009.
-
Rutkowski S, Bode U, Deinlein F, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352 (10): 978-86, 2005.
-
Albright AL, Wisoff JH, Zeltzer PM, et al.: Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neurosurgery 38 (2): 265-71, 1996.
-
Taylor RE, Bailey CC, Robinson K, et al.: Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 21 (8): 1581-91, 2003.
-
Lannering B, Rutkowski S, Doz F, et al.: Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 30 (26): 3187-93, 2012.
-
Thompson EM, Hielscher T, Bouffet E, et al.: Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 17 (4): 484-95, 2016.
-
Jakacki RI, Burger PC, Kocak M, et al.: Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: a report from the Children's Oncology Group. Pediatr Blood Cancer 62 (5): 776-83, 2015.
-
McManamy CS, Lamont JM, Taylor RE, et al.: Morphophenotypic variation predicts clinical behavior in childhood non-desmoplastic medulloblastomas. J Neuropathol Exp Neurol 62 (6): 627-32, 2003.
-
Massimino M, Antonelli M, Gandola L, et al.: Histological variants of medulloblastoma are the most powerful clinical prognostic indicators. Pediatr Blood Cancer 60 (2): 210-6, 2013.
-
Eberhart CG, Kratz J, Wang Y, et al.: Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 63 (5): 441-9, 2004.
-
Tabori U, Baskin B, Shago M, et al.: Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol 28 (8): 1345-50, 2010.
-
Onvani S, Etame AB, Smith CA, et al.: Genetics of medulloblastoma: clues for novel therapies. Expert Rev Neurother 10 (5): 811-23, 2010.
-
Dubuc AM, Northcott PA, Mack S, et al.: The genetics of pediatric brain tumors. Curr Neurol Neurosci Rep 10 (3): 215-23, 2010.
-
Giangaspero F, Wellek S, Masuoka J, et al.: Stratification of medulloblastoma on the basis of histopathological grading. Acta Neuropathol 112 (1): 5-12, 2006.
-
Jones DT, Jäger N, Kool M, et al.: Dissecting the genomic complexity underlying medulloblastoma. Nature 488 (7409): 100-5, 2012.
-
Peyrl A, Chocholous M, Kieran MW, et al.: Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer 59 (3): 511-7, 2012.
-
Kool M, Korshunov A, Remke M, et al.: Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123 (4): 473-84, 2012.
-
Schwalbe EC, Williamson D, Lindsey JC, et al.: DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol 125 (3): 359-71, 2013.
-
Polkinghorn WR, Tarbell NJ: Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol 4 (5): 295-304, 2007.
-
Ramaswamy V, Remke M, Bouffet E, et al.: Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol 14 (12): 1200-7, 2013.
-
Packer RJ, Zhou T, Holmes E, et al.: Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol 15 (1): 97-103, 2013.
-
Shaw DW, Geyer JR, Berger MS, et al.: Asymptomatic recurrence detection with surveillance scanning in children with medulloblastoma. J Clin Oncol 15 (5): 1811-3, 1997.
-
Torres CF, Rebsamen S, Silber JH, et al.: Surveillance scanning of children with medulloblastoma. N Engl J Med 330 (13): 892-5, 1994.
-
Kramer ED, Vezina LG, Packer RJ, et al.: Staging and surveillance of children with central nervous system neoplasms: recommendations of the Neurology and Tumor Imaging Committees of the Children's Cancer Group. Pediatr Neurosurg 20 (4): 254-62; discussion 262-3, 1994.
-
Perreault S, Lober RM, Carret AS, et al.: Surveillance imaging in children with malignant CNS tumors: low yield of spine MRI. J Neurooncol 116 (3): 617-23, 2014.
Cellular and Molecular Classification of CNS Embryonal TumorsMedulloblastoma By definition, medulloblastomas must arise in the posterior fossa.[1,2] The following four histologic types of medulloblastoma are recognized by the World Health Organization (WHO) classification:[2] - Medulloblastoma, classic.
- Medulloblastoma, desmoplastic/nodular.
- Medulloblastoma with extensive nodularity (MBEN).
- Medulloblastoma, large cell/anaplastic.
Significant attention has been focused on medulloblastomas that display anaplastic features, including increased nuclear size, marked cytological pleomorphism, numerous mitoses, and apoptotic bodies.[3,4] Using the criteria of anaplasia is subjective because most medulloblastomas have some degree of anaplasia. Foci of anaplasia may appear in tumors with histologic features of both classic and large cell medulloblastomas, and there is significant overlap between the anaplastic and large cell variant, which are frequently termed large cell/anaplastic medulloblastoma.[3,4] One convention is to consider medulloblastomas as anaplastic when anaplasia is diffuse (variably defined as anaplasia occurring in 50% to 80% of the tumor). The incidence of medulloblastoma with the desmoplastic/nodular histologic variant, which most commonly arises in a cerebellar hemisphere, is higher in infants, is less common in children, and increases again in adolescents and adults. The desmoplastic/nodular histologic variant is different from MBEN; the nodular variant has an expanded lobular architecture. The MBEN subtype occurs almost exclusively in infants and carries an excellent prognosis.[5,6] Subtypes of medulloblastoma Multiple medulloblastoma subtypes have been identified by integrative molecular analysis.[7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] Since 2012, the general consensus is that medulloblastoma can be molecularly separated into at least four core subtypes. These subtypes remain stable across primary and metastatic components.[23] It is likely that further subclassification will occur.[20,21,24] The 2016 World Health Organization (WHO) classification has endorsed this consensus by adding the following categories for genetically defined medulloblastoma:[2] - Medulloblastoma, WNT-activated.
- Medulloblastoma, sonic hedgehog (SHH)-activated and TP53-mutant.
- Medulloblastoma, SHH-activated and TP53-wildtype.
- Medulloblastoma, non-WNT/non-SHH.
The following four core molecularly defined subtypes of medulloblastoma have been identified:[20,21,25,26] - WNT medulloblastoma: WNT tumors are medulloblastomas with aberrations in the WNT signaling pathway. WNT medulloblastoma shows a WNT signaling gene expression signature and beta-catenin nuclear staining. They are usually histologically classified as classic medulloblastoma tumors and rarely have a large cell/anaplastic appearance. They are infrequently metastasized at diagnosis. Genetically, these tumors have 6q loss (monosomy 6), CTNNB1 mutations, and activated WNT signaling; MYC overexpression may be seen occasionally.[27]
The WNT subset is primarily observed in older children, adolescents, and adults and does not show a male predominance. The subset is believed to have brain stem origin, from the embryonal rhombic lip region. WNT medulloblastomas are associated with a very good outcome, especially in individuals whose tumors have beta-catenin nuclear staining and proven 6q loss and/or CTNNB1 mutations.[22,28] - SHH medulloblastoma: SHH tumors are medulloblastomas with aberrations in the SHH pathway. SHH medulloblastomas are characterized by chromosome 9q deletions; desmoplastic/nodular histology; and mutations in SHH pathway genes, including PTCH1, PTCH2, SMO, SUFU, and GLI2.
SHH medulloblastomas show a bimodal age distribution and are observed primarily in children younger than 3 years and in older adolescence/adulthood. The tumors are believed to emanate from the external granular layer of the cerebellum. Prognosis for patients with SHH medulloblastoma appears to be negatively affected by other molecular genetic changes, such as chromosome 17p loss, chromosome 3q gain, chromothripsis, p53 amplification, TP53 mutation, and the finding of large cell/anaplastic histology.[21,29] The outcome for patients with SHH medulloblastoma is relatively favorable, primarily in children younger than 3 years and in adults. This is likely because of the type of mutation present in the SHH pathway, given that patients with mutations upstream of the SHH signaling pathway, such as PTCH1, PTCH2, and SUFU, have a more favorable prognosis than do patients with downstream genomic aberrations, such as GLI2 and MYCN amplification.[30,31] Overall outcome in adolescents and young adults with SHH medulloblastoma is not different from that seen in patients with non-WNT pathway-activated tumors, except for patients with TP53 mutations and downstream SHH pathway mutations. Patients with unfavorable molecular findings have an unfavorable prognosis, with less than 50% of patients surviving after conventional treatment.[25,29,30,31,32] The 2016 WHO classification identifies SHH medulloblastoma with a TP53 mutation as a distinctive entity (medulloblastoma, SHH-activated and TP53-mutant).[2] Approximately 25% of SHH-activated medulloblastoma cases have TP53 mutations, with a high percentage of these cases also showing a TP53 germline mutation (9 of 20 in one study). These patients are commonly between the ages of 5 years and 18 years and have an inferior outcome (overall survival at 5 years, <50%).[29] The tumors often show large cell anaplastic histology.[29] - Group 3 medulloblastoma: Histology of group 3 medulloblastoma is either classic or large cell/anaplastic; these tumors are frequently metastasized at the time of diagnosis. A variety of different genomic aberrations have been noted in these tumors, including the presence of i17q and, most characteristically, MYC amplification.
Group 3 medulloblastomas occur throughout childhood and may occur in infants. Males outnumber females in a 2:1 ratio in this medulloblastoma subtype. Patients with group 3 medulloblastomas have a variable prognosis. Patients with MYC amplification or MYC overexpression have a poor prognosis, with less than 50% of these patients surviving 5 years after diagnosis. This poor prognosis is especially true in children younger than 4 years at diagnosis.[25] However, patients with group 3 medulloblastoma without MYC amplification or MYC overexpression who are older than 3 years have a prognosis similar to that of most patients with medulloblastoma, with a 5-year progression-free survival (PFS) rate higher than 70%.[32] - Group 4 medulloblastoma: Group 4 medulloblastomas are either classic or large cell/anaplastic tumors. Metastasis at diagnosis is common, but not as frequent as is seen in group 3 medulloblastomas. Molecularly, the medulloblastomas can have a CDK6 amplification, MYCN amplification, and most characteristically, an i17q abnormality.
Group 4 medulloblastomas occur throughout infancy and childhood and into adulthood. They also predominate in males. The prognosis is better than group 3 medulloblastoma but not as good as WNT medulloblastoma. Prognosis for group 4 medulloblastoma patients is affected by additional factors such as the presence of metastatic disease and chromosome 17p loss.[20,21]
Optimal ways of identifying the four core medulloblastoma subtypes for clinical use is under active study, and both immunohistochemical methods and methods based on gene expression analysis are under development.[28,33] The classification of medulloblastoma into the four major subtypes will be altered in the future.[34,35] Further subdivision within subgroups based on molecular characteristics is likely as each of the subgroups is further molecularly dissected, although there is no consensus regarding an alternative classification.[20,24,31] Whether the classification for adults with medulloblastoma has similar predictive ability in children is unknown.[21,25] In one study of adult medulloblastoma, MYC oncogene amplifications were rarely observed, and tumors with 6q deletion and WNT activation (as identified by nuclear beta-catenin staining) did not share the excellent prognosis seen in pediatric medulloblastomas, although another study did confirm an excellent prognosis for WNT-activated tumors in adults.[21,25] Nonmedulloblastoma Embryonal Tumors The WHO Classification of Tumors of the Central Nervous System (CNS) classifies nonmedulloblastoma embryonal tumors primarily by histologic and immunohistologic features, with the exception of embryonal tumor with multilayered rosettes (ETMR) and atypical teratoid tumor with rhabdoid features.[2] By definition, these tumors arise in the cerebral hemisphere, brain stem, or spinal cord and are composed of undifferentiated or poorly differentiated neuroepithelial cells that may display divergent differentiation. This classification, based on the histopathological characteristics and location of the tumor, is as follows: - ETMR, C19MC-altered.
- ETMR, not otherwise specified (NOS).
- Medulloepithelioma.
- CNS neuroblastoma.
- CNS ganglioneuroblastoma.
- CNS embryonal tumor, NOS.
- Atypical teratoid/rhabdoid tumor.
- CNS embryonal tumor with rhabdoid features.
CNS embryonal tumors that demonstrate distinct areas of neuronal differentiation are termed cerebral neuroblastomas and, if ganglion cells are present, ganglioneuroblastomas. Likewise, medulloepitheliomas have a specific histologic pattern and remain a separate entity.[2,36] Pineoblastoma is histologically similar to medulloblastoma and shares histologic features with embryonal tumors; however, because of the WHO classification, its histogenesis is linked to the pineocyte (a type of pineal cell) and is classified separately.[2] This classification does not take into account the molecular genetic makeup of these tumors.[2] Genomic molecular characterizations of embryonal tumors and pineoblastomas have demonstrated substantial heterogeneity among these tumors. These tumors are also molecularly different from medulloblastomas.[17] Although the WHO classification system does not yet use molecular findings to classify nonmedulloblastoma embryonal tumors, future classification will most likely be based on both histological and molecular findings and, possibly, site of origin in the nervous system. Subtypes of nonmedulloblastoma embryonal tumors A study applying unsupervised clustering of DNA methylation patterns for 323 nonmedulloblastoma embryonal tumors found that approximately one-half of these tumors diagnosed as nonmedulloblastoma embryonal tumors showed molecular profiles characteristic of other known pediatric brain tumors (e.g., high-grade glioma, atypical teratoid/rhabdoid tumor).[37] This observation highlights the utility of molecular characterization to assign this class of tumors to their appropriate biology-based diagnosis. Among the same collection of 323 tumors diagnosed as nonmedulloblastoma embryonal tumors, molecular characterization identified genomically and biologically distinctive subtypes, including the following: - Embryonal tumors with multilayered rosettes (ETMR): Representing 11% of the 323 cases, this subtype combines embryonal rosette-forming neuroepithelial brain tumors that were previously categorized as either embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, or medulloepithelioma.[37,38] ETMRs arise in young children (median age at diagnosis, 2-3 years) and show a highly aggressive clinical course, with a median PFS of less than 1 year and few long-term survivors.[38]
ETMRs are defined at the molecular level by high-level amplification of the microRNA cluster C19MC and by a gene fusion between TTYH1 and C19MC.[38,39,40] This gene fusion puts expression of C19MC under control of the TTYH1 promoter, leading to high-level aberrant expression of the microRNAs within the cluster. The World Health Organization (WHO) allows histologically similar tumors without C19MC alteration to be classified as ETMR. - CNS neuroblastoma with FOXR2 activation (CNS NB-FOXR2): Representing 14% of the 323 cases, this subtype is characterized by genomic alterations that lead to increased expression of the transcription factor FOXR2.[37] CNS NB-FOXR2 is primarily observed in children younger than 10 years, and the histology of these tumors is typically that of CNS neuroblastoma or CNS ganglioneuroblastoma .[37] There is no single genomic alteration among CNS NB-FOXR2 tumors leading to FOXR2 overexpression, with gene fusions involving multiple FOXR2 partners identified.[37] This subtype has not been added to the WHO diagnostic lexicon.
- CNS Ewing sarcoma family tumor with CIC alteration (CNS EFT-CIC): Representing 4% of the 323 cases, this subtype is characterized by genomic alterations affecting CIC (located on chromosome 19q13.2), with fusion to NUTM1 being identified in several cases tested.[37]CIC gene fusions are also identified in extra-CNS Ewing-like sarcomas, and the gene expression signature of CNS EFT-CIC tumors is similar to that of these sarcomas.[37] CNS EFT-CIC tumors generally occur in children younger than 10 years and are characterized by a small cell phenotype but with variable histology.[37] This subtype has not been added to the WHO diagnostic lexicon.
- CNS high-grade neuroepithelial tumor with MN1 alteration (CNS HGNET-MN1): Representing 3% of the 323 cases, this subtype is characterized by gene fusions involving MN1 (located on chromosome 22q12.3), with fusion partners including BEND2 and CXXC5.[37] This subtype shows a striking female predominance and tends to occur in the second decade of life.[37] This subtype contained most cases carrying a diagnosis of astroblastoma as per the 2007 WHO classification scheme.[37] This subtype has not been added to the WHO diagnostic lexicon.
- CNS high-grade neuroepithelial tumor with BCOR alteration (CNS HGNET-BCOR): Representing 3% of the 323 cases, this subtype is characterized by internal tandem duplications of BCOR,[37] a genomic alteration that is also found in clear cell sarcoma of the kidney.[41,42] While the median age at diagnosis is younger than 10 years, cases arising in the second decade of life and beyond do occur.[37] This subtype has not been added to the WHO diagnostic lexicon.
Medulloepithelioma Medulloepithelioma is identified as a histologically discrete tumor within the WHO classification system.[43,44] Medulloepithelioma tumors are rare and tend to arise most commonly in infants and young children. Medulloepitheliomas, which histologically recapitulate the embryonal neural tube, tend to arise supratentorially, primarily intraventricularly, but may arise infratentorially, in the cauda, and even extraneurally, along nerve roots.[43,44] Medulloepithelioma with the classic molecular change is considered an ETMR. Pineoblastoma Pineoblastoma, which was previously conventionally grouped with embryonal tumors, is now categorized by the WHO as a pineal parenchymal tumor. Given that therapies for pineoblastoma are quite similar to those utilized for embryonal tumors, the previous convention of including pineoblastoma with the CNS embryonal tumors is followed here. Pineoblastoma is associated with germline mutations in both the retinoblastoma (RB1) gene and in DICER1, as described below: - Pineoblastoma is associated with germline mutations in RB1, with the term trilateral retinoblastoma used to refer to ocular retinoblastoma in combination with a histologically similar brain tumor generally arising in the pineal gland or other midline structures. Historically, intracranial tumors have been reported in 5% to 15% of children with heritable retinoblastoma.[45] Rates of pineoblastoma among children with heritable retinoblastoma who undergo current treatment programs may be lower than these historical estimates.[46,47,48]
- Germline DICER1 mutations have also been reported in patients with pineoblastoma.[49] Among 18 patients with pineoblastoma, three patients with DICER1 germline mutations were identified, and an additional three patients known to be carriers of germline DICER1 mutations developed pineoblastoma.[49] The DICER1 mutations in patients with pineoblastoma are loss-of-function mutations that appear to be distinct from the mutations observed in DICER1 syndrome-related tumors such as pleuropulmonary blastoma.[49]
References:
-
Rorke LB: The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol 42 (1): 1-15, 1983.
-
Louis DN, Perry A, Reifenberger G, et al.: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6): 803-20, 2016.
-
McManamy CS, Lamont JM, Taylor RE, et al.: Morphophenotypic variation predicts clinical behavior in childhood non-desmoplastic medulloblastomas. J Neuropathol Exp Neurol 62 (6): 627-32, 2003.
-
Eberhart CG, Kratz J, Wang Y, et al.: Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 63 (5): 441-9, 2004.
-
Giangaspero F, Perilongo G, Fondelli MP, et al.: Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg 91 (6): 971-7, 1999.
-
Garrè ML, Cama A, Bagnasco F, et al.: Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome--a new clinical perspective. Clin Cancer Res 15 (7): 2463-71, 2009.
-
Onvani S, Etame AB, Smith CA, et al.: Genetics of medulloblastoma: clues for novel therapies. Expert Rev Neurother 10 (5): 811-23, 2010.
-
Dubuc AM, Northcott PA, Mack S, et al.: The genetics of pediatric brain tumors. Curr Neurol Neurosci Rep 10 (3): 215-23, 2010.
-
Thompson MC, Fuller C, Hogg TL, et al.: Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24 (12): 1924-31, 2006.
-
Kool M, Koster J, Bunt J, et al.: Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3 (8): e3088, 2008.
-
Tabori U, Baskin B, Shago M, et al.: Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol 28 (8): 1345-50, 2010.
-
Pfister S, Remke M, Benner A, et al.: Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol 27 (10): 1627-36, 2009.
-
Ellison DW, Onilude OE, Lindsey JC, et al.: beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol 23 (31): 7951-7, 2005.
-
Polkinghorn WR, Tarbell NJ: Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol 4 (5): 295-304, 2007.
-
Giangaspero F, Wellek S, Masuoka J, et al.: Stratification of medulloblastoma on the basis of histopathological grading. Acta Neuropathol 112 (1): 5-12, 2006.
-
Northcott PA, Korshunov A, Witt H, et al.: Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29 (11): 1408-14, 2011.
-
Pomeroy SL, Tamayo P, Gaasenbeek M, et al.: Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415 (6870): 436-42, 2002.
-
Jones DT, Jäger N, Kool M, et al.: Dissecting the genomic complexity underlying medulloblastoma. Nature 488 (7409): 100-5, 2012.
-
Peyrl A, Chocholous M, Kieran MW, et al.: Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer 59 (3): 511-7, 2012.
-
Taylor MD, Northcott PA, Korshunov A, et al.: Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123 (4): 465-72, 2012.
-
Kool M, Korshunov A, Remke M, et al.: Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123 (4): 473-84, 2012.
-
Pietsch T, Schmidt R, Remke M, et al.: Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol 128 (1): 137-49, 2014.
-
Wang X, Dubuc AM, Ramaswamy V, et al.: Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol 129 (3): 449-57, 2015.
-
Northcott PA, Jones DT, Kool M, et al.: Medulloblastomics: the end of the beginning. Nat Rev Cancer 12 (12): 818-34, 2012.
-
Cho YJ, Tsherniak A, Tamayo P, et al.: Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29 (11): 1424-30, 2011.
-
Gajjar A, Bowers DC, Karajannis MA, et al.: Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol 33 (27): 2986-98, 2015.
-
Ellison DW, Kocak M, Dalton J, et al.: Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol 29 (11): 1400-7, 2011.
-
Ellison DW, Dalton J, Kocak M, et al.: Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121 (3): 381-96, 2011.
-
Zhukova N, Ramaswamy V, Remke M, et al.: Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol 31 (23): 2927-35, 2013.
-
Shih DJ, Northcott PA, Remke M, et al.: Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol 32 (9): 886-96, 2014.
-
Kool M, Jones DT, Jäger N, et al.: Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 25 (3): 393-405, 2014.
-
Schwalbe EC, Williamson D, Lindsey JC, et al.: DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol 125 (3): 359-71, 2013.
-
Northcott PA, Shih DJ, Remke M, et al.: Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol 123 (4): 615-26, 2012.
-
Gottardo NG, Hansford JR, McGlade JP, et al.: Medulloblastoma Down Under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol 127 (2): 189-201, 2014.
-
Louis DN, Perry A, Burger P, et al.: International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 24 (5): 429-35, 2014.
-
Benesch M, Sperl D, von Bueren AO, et al.: Primary central nervous system primitive neuroectodermal tumors (CNS-PNETs) of the spinal cord in children: four cases from the German HIT database with a critical review of the literature. J Neurooncol 104 (1): 279-86, 2011.
-
Sturm D, Orr BA, Toprak UH, et al.: New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 164 (5): 1060-72, 2016.
-
Korshunov A, Sturm D, Ryzhova M, et al.: Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol 128 (2): 279-89, 2014.
-
Kleinman CL, Gerges N, Papillon-Cavanagh S, et al.: Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet 46 (1): 39-44, 2014.
-
Li M, Lee KF, Lu Y, et al.: Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell 16 (6): 533-46, 2009.
-
Ueno-Yokohata H, Okita H, Nakasato K, et al.: Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet 47 (8): 861-3, 2015.
-
Roy A, Kumar V, Zorman B, et al.: Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun 6: 8891, 2015.
-
Louis DN, Ohgaki H, Wiestler OD, et al.: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114 (2): 97-109, 2007.
-
Sharma MC, Mahapatra AK, Gaikwad S, et al.: Pigmented medulloepithelioma: report of a case and review of the literature. Childs Nerv Syst 14 (1-2): 74-8, 1998 Jan-Feb.
-
de Jong MC, Kors WA, de Graaf P, et al.: Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 15 (10): 1157-67, 2014.
-
Ramasubramanian A, Kytasty C, Meadows AT, et al.: Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol 156 (4): 825-9, 2013.
-
Abramson DH, Dunkel IJ, Marr BP, et al.: Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol 156 (6): 1319-20, 2013.
-
Turaka K, Shields CL, Meadows AT, et al.: Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer 59 (1): 121-5, 2012.
-
de Kock L, Sabbaghian N, Druker H, et al.: Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol 128 (4): 583-95, 2014.
Stage Information for CNS Embryonal TumorsStaging of Medulloblastoma Historically, staging was based on an intraoperative evaluation of both the size and extent of the tumor, coupled with postoperative neuroimaging of the brain and spine and cytological evaluation of cerebrospinal fluid (CSF) (the Chang system). Intraoperative evaluation of the extent of the tumor has been supplanted by neuraxis imaging before diagnosis and postoperative imaging to determine amount of primary site residual disease. The following tests and procedures are now used for staging: - Magnetic resonance imaging (MRI) of the brain and spine (often done preoperatively).
- Postoperative MRI of the brain to determine the amount of residual disease.
- Lumbar CSF analysis.[1,2,3]
The tumor extent is defined as: - M0: No dissemination.
- M1: CSF-positive cytology only.
- M2: Gross nodular seeding in cerebellar-cerebral subarachnoid space and/or lateral or third ventricle.
- M3: Gross nodular seeding in spinal subarachnoid space.
- M4: Extraneural metastasis.
Postoperative degree of residual disease is designated as: - Gross-total resection/near-total resection: No or minimal (not measurable) evidence of residual disease after diagnosis.
- Subtotal resection: Residual disease after diagnosis; this is conventionally further subdivided into less than, more than, or equal to 1.5 cm2 of measurable residual disease.
- Biopsy: No tumor resection; only a sample of tumor tissue is removed.
Since the 1990s, prospective studies have been performed using this staging system to separate patients into average-risk and high-risk medulloblastoma subgroups.[2,3,4] The presence of diffuse (>50% of the pathologic specimen) histologic anaplasia has been incorporated as an addition to staging systems. If diffuse anaplasia is found, patients with otherwise average-risk disease are upstaged to high-risk disease. Staging of Nonmedulloblastoma Embryonal Tumors Patients with nonmedulloblastoma, nonmedulloepithelioma embryonal tumors are staged in a fashion similar to that used for children with medulloblastoma; however, the patients are not assigned to average-risk and high-risk subgroups for treatment purposes (refer to the Staging of Medulloblastoma section of this summary for more information). Medulloepitheliomas frequently disseminate to the neuraxis.[5] Medulloepithelioma is staged in the same way as medulloblastoma; however, the patients are not assigned to average-risk and high-risk subgroups for treatment purposes (refer to the Staging of Medulloblastoma section of this summary for more information). Staging of Pineoblastoma Dissemination at the time of diagnosis occurs in 10% to 30% of patients.[6] Because of the location of the tumor, total resections are uncommon, and most patients have only a biopsy or a subtotal resection before postsurgical treatment.[6,7] Staging for children with pineoblastomas is the same as that performed for children with medulloblastoma; however, the patients are not assigned to average-risk and high-risk subgroups for treatment purposes (refer to the Staging of Medulloblastoma section of this summary for more information).[6] References:
-
Fouladi M, Gajjar A, Boyett JM, et al.: Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 17 (10): 3234-7, 1999.
-
Zeltzer PM, Boyett JM, Finlay JL, et al.: Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol 17 (3): 832-45, 1999.
-
Yao MS, Mehta MP, Boyett JM, et al.: The effect of M-stage on patterns of failure in posterior fossa primitive neuroectodermal tumors treated on CCG-921: a phase III study in a high-risk patient population. Int J Radiat Oncol Biol Phys 38 (3): 469-76, 1997.
-
Taylor RE, Bailey CC, Robinson K, et al.: Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 21 (8): 1581-91, 2003.
-
Müller K, Zwiener I, Welker H, et al.: Curative treatment for central nervous system medulloepithelioma despite residual disease after resection. Report of two cases treated according to the GPHO Protocol HIT 2000 and review of the literature. Strahlenther Onkol 187 (11): 757-62, 2011.
-
Jakacki RI, Zeltzer PM, Boyett JM, et al.: Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol 13 (6): 1377-83, 1995.
-
Timmermann B, Kortmann RD, Kühl J, et al.: Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol 20 (3): 842-9, 2002.
Treatment Option Overview for CNS Embryonal TumorsRisk Stratification for Medulloblastoma Risk stratification is based on neuroradiographic evaluation for disseminated disease, cerebrospinal fluid (CSF) cytological examination, postoperative neuroimaging evaluation for the amount of residual disease, and patient age. Patients older than 3 years with medulloblastoma have been stratified into the following two risk groups: - Average risk: Children older than 3 years with tumors that are totally resected or near-totally resected (≤1.5 cm2 of residual disease) and have no metastatic disease.[1]
- High risk: Children older than 3 years with metastatic disease and/or subtotal resection (>1.5 cm2 of residual disease).[1] Metastatic disease includes neuroradiographic evidence of disseminated disease, positive cytology in lumbar or ventricular CSF obtained more than 10 days after surgery, or extraneural disease.[1] Children with tumors showing diffuse anaplasia and who otherwise would have been considered average risk, are assigned to the high-risk group.[2,3]
For younger children, in some studies for those younger than 3 years and for others younger than 4 or 5 years, similar separation into average-risk (no dissemination and ≤1.5 cm2 of residual disease) or high-risk (disseminated disease and/or >1.5 cm2 of residual disease) groups has been employed. Histologic findings of desmoplasia have also been used to connote a more favorable risk subgrouping, especially for the medulloblastoma with extensive nodularity subgroup.[4,5] Assigning a risk group based on extent of resection and disease at diagnosis may not predict treatment outcome. Molecular genetics and histologic factors may be more informative, although they must be evaluated in the context of the age of the patient, extent of disease at time of diagnosis, and treatment received.[6,7] Although molecular subdivisions will likely change risk characterization in the future,[8] they are not routinely used to assign treatment in North American prospective studies (e.g., NCT01878617). Table 3. Standard Treatment Options for Childhood Central Nervous System (CNS) Embryonal Tumors| Treatment Group | Standard Treatment Options |
|---|
| Newly diagnosed childhood medulloblastoma: | | | | Children older than 3 years with average-risk medulloblastoma | Surgery | | Adjuvant therapy(radiation therapy and chemotherapy) | | | Children older than 3 years with high-risk medulloblastoma | Surgery | | Adjuvant therapy(radiation therapy and chemotherapy) | | | Children aged 3 years and younger | Surgery | | Adjuvant chemotherapy | | | | Newly diagnosed nonmedulloblastoma, nonmedulloepithelioma embryonal tumor: | | | | Children older than 3 years | Surgery | | Adjuvant therapy(radiation therapy and chemotherapy) | | | Children aged 3 years and younger | Similar to children aged 3 years and younger with medulloblastoma (surgery and adjuvant chemotherapy) | | | | Newly diagnosed medulloepithelioma | Same as for children with high-risk medulloblastoma (surgery and adjuvant therapy) and children aged 3 years and younger with other embryonal tumors | | | | Newly diagnosed pineoblastoma: | | | | Children older than 3 years | Surgery | | Adjuvant therapy(radiation therapy and chemotherapy) | | | Children aged 3 years and younger | Biopsy(for diagnosis) | | Chemotherapy | | High-dose, marrow-ablative chemotherapy with autologous bone marrow rescue or peripheral stem cell rescue | | | | Recurrent childhood CNS embryonal tumors (standard treatment options not defined) | Surgery | | Radiation therapy | | Chemotherapy | | High-dose chemotherapy with stem cell rescue | | Molecularly targeted therapy | (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment for more information about the treatment of CNS atypical teratoid/rhabdoid tumors.) References:
-
Zeltzer PM, Boyett JM, Finlay JL, et al.: Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol 17 (3): 832-45, 1999.
-
Giangaspero F, Wellek S, Masuoka J, et al.: Stratification of medulloblastoma on the basis of histopathological grading. Acta Neuropathol 112 (1): 5-12, 2006.
-
Eberhart CG, Kratz J, Wang Y, et al.: Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 63 (5): 441-9, 2004.
-
Rutkowski S, Bode U, Deinlein F, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352 (10): 978-86, 2005.
-
Rutkowski S, Gerber NU, von Hoff K, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol 11 (2): 201-10, 2009.
-
Taylor MD, Northcott PA, Korshunov A, et al.: Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123 (4): 465-72, 2012.
-
Ramaswamy V, Remke M, Adamski J, et al.: Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro Oncol 18 (2): 291-7, 2016.
-
Gottardo NG, Hansford JR, McGlade JP, et al.: Medulloblastoma Down Under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol 127 (2): 189-201, 2014.
Treatment of Newly Diagnosed Childhood MedulloblastomaTreatment Modalities Surgery Surgery is considered a standard part of treatment for histologic confirmation of tumor type and as a means to improve outcome. Total or near-total resections are considered optimal, if they can be performed safely.[1,2] Postoperatively, children may have significant neurologic deficits caused by preoperative tumor-related brain injury, hydrocephalus, or surgery-related brain injury.[3][Level of evidence: 3iC] A significant number of patients with medulloblastoma will develop cerebellar mutism syndrome (also known as posterior fossa syndrome). Symptoms of cerebellar mutism syndrome include the following: - Delayed onset of speech.
- Suprabulbar palsies.
- Ataxia.
- Hypotonia.
- Emotional lability.
The etiology of cerebellar mutism syndrome remains unclear, although cerebellar vermian damage and/or disruption of cerebellar-cortical tracts has been postulated as the possible cause for the mutism.[4,5]; [6][Level of evidence: 3iC] In two Children's Cancer Group studies evaluating children with both average-risk and high-risk medulloblastoma, the syndrome has been identified in nearly 25% of patients.[5,6,7]; [8][Level of evidence: 3iiiC] Approximately 50% of patients with this syndrome manifest long-term, permanent neurologic and neurocognitive sequelae.[6,8] Radiation therapy Radiation therapy to the primary tumor site is usually in the range of 54 Gy to 55.8 Gy. In most instances, this is given with a margin of 1 cm to 2 cm around the primary tumor site, preferably by conformal techniques. For all medulloblastomas in children older than 3 or 4 years at diagnosis, craniospinal radiation therapy is given at doses ranging between 23.4 Gy and 36 Gy, depending on risk factors such as extent of disease at diagnosis. A prospective phase II toxicity study of proton radiation therapy [9] and a retrospective efficacy report of protons versus photons for medulloblastoma [10] demonstrated equivalent outcomes for progression-free survival (PFS), overall survival (OS), patterns of relapse, and delayed toxic effects. Comparative outcomes for these treatment technologies are under investigation. Chemotherapy is routinely administered during and after radiation therapy. For children younger than 3 years, efforts are made to omit or delay radiation, given the profound impact of radiation at this age. Children of all ages are susceptible to the adverse effects of radiation on brain development. Debilitating effects on neurologic/cognitive development, growth, and endocrine function have been frequently observed, especially in younger children.[11,12,13,14,15] Chemotherapy Chemotherapy, usually given during and after radiation therapy, is a standard component of treatment for older children with medulloblastoma and other embryonal tumors. Chemotherapy can be used to delay and sometimes obviate the need for radiation therapy in 20% to 40% of children younger than 3 to 4 years with nondisseminated medulloblastoma.[16,17]; [15][Level of evidence: 3iiiC] Children Older Than 3 Years with Average-Risk Medulloblastoma Standard treatment options Standard treatment options for children older than 3 years with newly diagnosed average-risk medulloblastoma include the following: - Surgery.
- Adjuvant therapy.
- Radiation therapy.
- Chemotherapy.
Surgery If deemed feasible, total or near-total removal of the tumor is considered optimal.[1] Adjuvant therapy Radiation therapy is usually initiated after surgery with or without concurrent chemotherapy.[18,19,20] Adjuvant radiation therapy - The best survival results for children with medulloblastoma have been obtained when radiation therapy is initiated within 4 to 6 weeks postsurgery.[19,20,21]; [22,23][Level of evidence: 1iA]
- The radiation dose for patients with average-risk medulloblastoma is 54 Gy to 55 Gy to the posterior fossa or local tumor bed and 23.4 Gy to the entire neuraxis (i.e., the whole brain and spine).[18,19,20,24]
- With radiation therapy alone, 5-year event-free survival (EFS) rates range between 50% and 65% in those with nondisseminated disease.[19,25]
- The minimal dose of craniospinal radiation needed for disease control is unknown. Attempts to lower the dose of craniospinal radiation therapy to 23.4 Gy without chemotherapy have resulted in an increased incidence of isolated leptomeningeal relapse.[24]
- If chemotherapy is added after radiation therapy, 23.4 Gy of craniospinal radiation therapy has been shown to be an effective dose.[23,26,27] Lower doses are being evaluated.
- Although the standard boost in medulloblastoma is to the entire posterior fossa, failure data patterns suggest that radiation therapy to the tumor bed instead of the entire posterior fossa would be equally effective and may be associated with reduced toxicity.[28,29]
Adjuvant chemotherapy Chemotherapy is now a standard component of the treatment of children with average-risk medulloblastoma. - Prospective randomized trials and single-arm trials suggest that adjuvant chemotherapy given during and after radiation therapy improves OS for children with average-risk medulloblastoma.[8,18,19,20,21,22]
- Radiation therapy and chemotherapy given during and after surgery has demonstrated 5-year EFS rates of 70% to 85%.[18,19,20]; [30][Level of evidence: 2A]
- A lower radiation dose of 23.4 Gy to the neuraxis when coupled with chemotherapy has been shown to result in disease control in up to 85% of patients and may decrease the severity of long-term neurocognitive sequelae.[23,26,27,31]
- A variety of chemotherapeutic regimens have been successfully used, including the combination of cisplatin, lomustine, and vincristine or the combination of cisplatin, cyclophosphamide, and vincristine.[18,19,31,32] In addition, postradiation, high-dose cyclophosphamide supported by peripheral stem cell rescue, but with reduced cumulative doses of vincristine and cisplatin, has resulted in similar survival rates.[33]
- Although medulloblastoma is often sensitive to chemotherapy, preradiation chemotherapy has not been shown to improve survival compared with treatment with radiation therapy and subsequent chemotherapy. In some prospective studies, preradiation chemotherapy has been related to a poorer rate of survival.[19,20,21,22]
Children Older Than 3 Years with High-Risk Medulloblastoma Standard treatment options Standard treatment options for children older than 3 years who are newly diagnosed with medulloblastoma and have metastatic disease or have had a subtotal resection include the following: - Surgery.
- Adjuvant therapy.
- Radiation therapy.
- Chemotherapy.
Surgery As for those with average-risk disease, attempt at gross-total resection is considered optimal, if deemed feasible.[1,25] Adjuvant therapy In high-risk patients, numerous studies have demonstrated that multimodality therapy improves the duration of disease control and overall disease-free survival (DFS).[33,34] Studies show that approximately 50% to 65% of patients with high-risk disease will experience long-term disease control.[18,33,34,35,36]; [37][Level of evidence: 1iiA] Adjuvant radiation therapy - In contrast to standard-risk treatment, the craniospinal radiation dose is generally 36 Gy.
Adjuvant chemotherapy - The drugs that have been found to be useful in children with average-risk disease are the same drugs that have been used extensively in children with high-risk disease, including cisplatin, lomustine, cyclophosphamide, etoposide, and vincristine.[37]
- Postradiation, high-dose, nonmyeloablative chemotherapy supported by peripheral stem cell rescue, but with reduced cumulative doses of vincristine and cisplatin, has also been utilized and has resulted in 5-year PFS rates of approximately 60%.[33]
Treatment options under clinical evaluation The following is an example of a national and/or institutional clinical trial that is currently being conducted. Information about ongoing clinical trials is available from the NCI website. - COG-ACNS0332 (NCT00392327) (Chemotherapy and Radiation Therapy in Treating Young Patients With Newly Diagnosed, Previously Untreated, High-Risk Medulloblastoma or Supratentorial Primitive Neuroectodermal Tumor [PNET]): This COG phase III trial for children older than 3 years is evaluating the efficacy of adding carboplatin to radiation therapy with vincristine, followed by maintenance chemotherapy with conventional adjuvant chemotherapy and isotretinoin. This trial is closed to patients with supratentorial PNETs.
Children Aged 3 Years and Younger Standard treatment options Five-year DFS rates for young children with medulloblastoma have ranged between 30% and 70%, with most long-term survivors successfully treated with chemotherapy alone, having nondisseminated, totally resected tumors and histologic evidence of desmoplasia.[16,38,39]; [40][Level of evidence: 2A] The treatment of children younger than 3 to 4 years with newly diagnosed medulloblastoma continues to evolve. Therapeutic approaches have attempted to delay and, in some cases, avoid the use of craniospinal radiation therapy because of its deleterious effects on the immature nervous system. Results have been variable, and comparison across studies has been difficult because of differences in drug regimens used and the utilization of craniospinal and local boost radiation therapy at the end of chemotherapy or when children reached age 3 years in some studies. Standard treatment options for children aged 3 years and younger with newly diagnosed medulloblastoma include the following: - Surgery.
- Adjuvant chemotherapy.
Surgery If deemed feasible, complete surgical resection of the tumor is the optimal treatment. Surgical resectability is associated with histology, as patients with desmoplastic/nodular medulloblastoma or medulloblastoma with extensive nodularity (MBEN) have a higher rate of complete resection than do patients with classic medulloblastoma.[41,42] Adjuvant chemotherapy - Therapies for younger children with medulloblastoma have included the use of multiagent chemotherapeutic approaches, including drugs such as cyclophosphamide, etoposide, cisplatin, and vincristine, with or without concomitant high-dose intravenous methotrexate and/or intrathecal methotrexate or mafosfamide, and/or intraventricular methotrexate.[16,38,39,42,43,44]; [45][Level of evidence: 2A]; [46][Level of evidence: 2B]
- Several studies have observed that the histologic finding of desmoplasia, seen in patients with desmoplastic medulloblastoma or MBEN, connotes a significantly better prognosis compared with outcome for patients with classic or large cell/anaplastic medulloblastoma.[41,42,47,48,49]; [50][Level of evidence: 2A]
- Desmoplasia was an independent predictor of favorable EFS rates in the German HIrnTumor (HIT) 2000 multicenter trial in which 19 patients with desmoplastic medulloblastoma or MBEN had 5-year EFS rates of 90% ± 7% and OS rates of 100% ± 0%, with all patients being treated with postoperative chemotherapy alone (including intraventricular methotrexate) before progression.[42]
- By contrast, EFS and OS rates for children with classic medulloblastoma in the HIT 2000 trial were significantly lower (EFS, 30% ± 11%; OS, 68% ± 10%).[42]
- The COG clinical trial CCG-9921 also observed a favorable outcome for children with desmoplastic medulloblastoma (including MBEN), with an EFS of 77% ± 9% and an OS of 85% ± 8% for the desmoplastic group compared with an EFS of 17% ± 5% and OS of 29% ± 6% for patients in the nondesmoplastic group (P < .0001 for both EFS and OS comparisons).[16] In this study, patients with desmoplastic tumors did not receive radiation therapy before progression.
- Compared with children with desmoplastic medulloblastoma or MBEN treated with current intensive chemotherapy regimens, children with other histologic subtypes fare less well.
- EFS rates are below 40% despite the use of intensive chemotherapy supplemented with methotrexate (intravenous, intrathecal, and intraventricular) and the use of high-dose chemotherapeutic regimens supported with stem cell rescue.[16,42,51]
- Outcome is particularly poor when these patients have disseminated disease. There is no consensus on when and how much radiation therapy should be given and at what age radiation therapy should be instituted in patients with disseminated disease.[16,38,39,51]
- Another treatment option for children younger than 3 years at diagnosis is chemotherapy followed by autologous stem cell rescue. Results of trials utilizing higher-dose, marrow-ablative chemotherapeutic regimens supported by stem cell rescue have also demonstrated that a subgroup of patients with medulloblastoma who are younger than 3 years at the time of diagnosis can be treated with chemotherapy alone.[17,40,52][Level of evidence: 2A] However, in some studies, radiation to the primary tumor site and/or craniospinal axis has been added after chemotherapy, making assessment of the efficacy of chemotherapy more difficult.[51]
Current Clinical Trials Check the list of NCI-supported cancer clinical trials that are now accepting patients with untreated childhood medulloblastoma. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. References:
-
Albright AL, Wisoff JH, Zeltzer PM, et al.: Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neurosurgery 38 (2): 265-71, 1996.
-
Thompson EM, Hielscher T, Bouffet E, et al.: Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 17 (4): 484-95, 2016.
-
Stargatt R, Rosenfeld JV, Maixner W, et al.: Multiple factors contribute to neuropsychological outcome in children with posterior fossa tumors. Dev Neuropsychol 32 (2): 729-48, 2007.
-
Pollack IF, Polinko P, Albright AL, et al.: Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 37 (5): 885-93, 1995.
-
Robertson PL, Muraszko KM, Holmes EJ, et al.: Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg 105 (6): 444-51, 2006.
-
Wells EM, Khademian ZP, Walsh KS, et al.: Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr 5 (4): 329-34, 2010.
-
Gudrunardottir T, Sehested A, Juhler M, et al.: Cerebellar mutism: review of the literature. Childs Nerv Syst 27 (3): 355-63, 2011.
-
Wolfe-Christensen C, Mullins LL, Scott JG, et al.: Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer 49 (5): 723-6, 2007.
-
Yock TI, Yeap BY, Ebb DH, et al.: Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 17 (3): 287-98, 2016.
-
Eaton BR, Esiashvili N, Kim S, et al.: Clinical Outcomes Among Children With Standard-Risk Medulloblastoma Treated With Proton and Photon Radiation Therapy: A Comparison of Disease Control and Overall Survival. Int J Radiat Oncol Biol Phys 94 (1): 133-8, 2016.
-
Ris MD, Packer R, Goldwein J, et al.: Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol 19 (15): 3470-6, 2001.
-
Packer RJ, Sutton LN, Atkins TE, et al.: A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg 70 (5): 707-13, 1989.
-
Johnson DL, McCabe MA, Nicholson HS, et al.: Quality of long-term survival in young children with medulloblastoma. J Neurosurg 80 (6): 1004-10, 1994.
-
Walter AW, Mulhern RK, Gajjar A, et al.: Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol 17 (12): 3720-8, 1999.
-
Laughton SJ, Merchant TE, Sklar CA, et al.: Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol 26 (7): 1112-8, 2008.
-
Geyer JR, Sposto R, Jennings M, et al.: Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol 23 (30): 7621-31, 2005.
-
Chi SN, Gardner SL, Levy AS, et al.: Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol 22 (24): 4881-7, 2004.
-
Packer RJ, Sutton LN, Elterman R, et al.: Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg 81 (5): 690-8, 1994.
-
Bailey CC, Gnekow A, Wellek S, et al.: Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol 25 (3): 166-78, 1995.
-
Kortmann RD, Kühl J, Timmermann B, et al.: Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys 46 (2): 269-79, 2000.
-
Taylor RE, Bailey CC, Robinson KJ, et al.: Impact of radiotherapy parameters on outcome in the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int J Radiat Oncol Biol Phys 58 (4): 1184-93, 2004.
-
von Hoff K, Hinkes B, Gerber NU, et al.: Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. Eur J Cancer 45 (7): 1209-17, 2009.
-
Packer RJ, Gajjar A, Vezina G, et al.: Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24 (25): 4202-8, 2006.
-
Thomas PR, Deutsch M, Kepner JL, et al.: Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol 18 (16): 3004-11, 2000.
-
Taylor RE, Bailey CC, Robinson K, et al.: Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 21 (8): 1581-91, 2003.
-
Oyharcabal-Bourden V, Kalifa C, Gentet JC, et al.: Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. J Clin Oncol 23 (21): 4726-34, 2005.
-
Merchant TE, Kun LE, Krasin MJ, et al.: Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 70 (3): 782-7, 2008.
-
Fukunaga-Johnson N, Sandler HM, Marsh R, et al.: The use of 3D conformal radiotherapy (3D CRT) to spare the cochlea in patients with medulloblastoma. Int J Radiat Oncol Biol Phys 41 (1): 77-82, 1998.
-
Huang E, Teh BS, Strother DR, et al.: Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 52 (3): 599-605, 2002.
-
Carrie C, Grill J, Figarella-Branger D, et al.: Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol 27 (11): 1879-83, 2009.
-
Packer RJ, Goldwein J, Nicholson HS, et al.: Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol 17 (7): 2127-36, 1999.
-
Nageswara Rao AA, Wallace DJ, Billups C, et al.: Cumulative cisplatin dose is not associated with event-free or overall survival in children with newly diagnosed average-risk medulloblastoma treated with cisplatin based adjuvant chemotherapy: report from the Children's Oncology Group. Pediatr Blood Cancer 61 (1): 102-6, 2014.
-
Gajjar A, Chintagumpala M, Ashley D, et al.: Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7 (10): 813-20, 2006.
-
Verlooy J, Mosseri V, Bracard S, et al.: Treatment of high risk medulloblastomas in children above the age of 3 years: a SFOP study. Eur J Cancer 42 (17): 3004-14, 2006.
-
Gandola L, Massimino M, Cefalo G, et al.: Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol 27 (4): 566-71, 2009.
-
Evans AE, Jenkin RD, Sposto R, et al.: The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg 72 (4): 572-82, 1990.
-
Jakacki RI, Burger PC, Zhou T, et al.: Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol 30 (21): 2648-53, 2012.
-
Grill J, Sainte-Rose C, Jouvet A, et al.: Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol 6 (8): 573-80, 2005.
-
Rutkowski S, Bode U, Deinlein F, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352 (10): 978-86, 2005.
-
Cohen BH, Geyer JR, Miller DC, et al.: Pilot Study of Intensive Chemotherapy With Peripheral Hematopoietic Cell Support for Children Less Than 3 Years of Age With Malignant Brain Tumors, the CCG-99703 Phase I/II Study. A Report From the Children's Oncology Group. Pediatr Neurol 53 (1): 31-46, 2015.
-
Rutkowski S, von Hoff K, Emser A, et al.: Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 28 (33): 4961-8, 2010.
-
von Bueren AO, von Hoff K, Pietsch T, et al.: Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 13 (6): 669-79, 2011.
-
Duffner PK, Horowitz ME, Krischer JP, et al.: Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328 (24): 1725-31, 1993.
-
Ater JL, van Eys J, Woo SY, et al.: MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol 32 (3): 243-52, 1997.
-
Grundy RG, Wilne SH, Robinson KJ, et al.: Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer 46 (1): 120-33, 2010.
-
Blaney SM, Kocak M, Gajjar A, et al.: Pilot study of systemic and intrathecal mafosfamide followed by conformal radiation for infants with intracranial central nervous system tumors: a pediatric brain tumor consortium study (PBTC-001). J Neurooncol 109 (3): 565-71, 2012.
-
Leary SE, Zhou T, Holmes E, et al.: Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children's oncology group. Cancer 117 (14): 3262-7, 2011.
-
Giangaspero F, Perilongo G, Fondelli MP, et al.: Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg 91 (6): 971-7, 1999.
-
Garrè ML, Cama A, Bagnasco F, et al.: Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome--a new clinical perspective. Clin Cancer Res 15 (7): 2463-71, 2009.
-
Rutkowski S, Gerber NU, von Hoff K, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol 11 (2): 201-10, 2009.
-
Lafay-Cousin L, Smith A, Chi SN, et al.: Clinical, Pathological, and Molecular Characterization of Infant Medulloblastomas Treated with Sequential High-Dose Chemotherapy. Pediatr Blood Cancer 63 (9): 1527-34, 2016.
-
Dhall G, Grodman H, Ji L, et al.: Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the "Head Start" I and II protocols. Pediatr Blood Cancer 50 (6): 1169-75, 2008.
Treatment of Newly Diagnosed Nonmedulloblastoma Embryonal Tumors(Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment for more information about the treatment of central nervous system [CNS] atypical teratoid/rhabdoid tumors.) (Refer to the Treatment of Newly Diagnosed Medulloepithelioma section of this summary for information about the treatment of medulloepithelioma.) Children Older Than 3 Years Standard treatment options Standard treatment options for children older than 3 years with newly diagnosed nonmedulloblastoma, nonmedulloepithelioma embryonal tumors include the following: - Surgery.
- Adjuvant therapy.
- Radiation therapy.
- Chemotherapy.
Surgery - Attempting aggressive surgical resection is the first step in the management of newly diagnosed nonmedulloblastoma embryonal tumors. Although previous studies did not demonstrate that the extent of resection is predictive of outcome,[1,2,3] one study demonstrated an improved survival when the tumor was completely resected.[4][Level of evidence: 2A]
- Nonmedulloblastoma embryonal tumors are often amenable to resection; in reported case series, 50% to 60% of patients were totally or near-totally resected.[1,2]
Adjuvant therapy After surgery, children with nonmedulloblastoma embryonal tumors usually receive treatment similar to that received by children with high-risk medulloblastoma. Adjuvant radiation therapy and chemotherapy - Conventionally, patients are treated with radiation to the entire neuraxis with local boost radiation therapy, as given for medulloblastoma.[3] However, the local boost radiation therapy may be problematic because of the size of the tumor and its location in the cerebral cortex. Also, there is no definitive evidence that craniospinal radiation therapy is superior to radiation to the primary tumor site alone in children with nondisseminated lesions.[1,2,3]
- The chemotherapeutic approaches during and after radiation therapy are similar to those used for children with high-risk medulloblastoma. Three-year to 5-year overall survival rates of 25% to 50% have been noted.[1,2,3]; [4,5][Level of evidence: 2A]; [6][Level of evidence: 3iiiB]
Children Aged 3 Years and Younger Standard treatment options Treatment of children aged 3 years and younger with nonmedulloblastoma, nonmedulloepithelioma embryonal tumors is similar to that outlined for children aged 3 years and younger with medulloblastoma. (Refer to the medulloblastoma Children Aged 3 Years and Younger section of this summary for more information). With the use of chemotherapy alone, outcome has been variable, with survival rates at 5 years ranging between 0% and 50%.[7,8,9]; [10][Level of evidence: 2Di] The addition of craniospinal irradiation to chemotherapy-based regimens may successfully treat some children but with anticipated neurodevelopmental decline.[11][Level of evidence: 2A] References:
-
Cohen BH, Zeltzer PM, Boyett JM, et al.: Prognostic factors and treatment results for supratentorial primitive neuroectodermal tumors in children using radiation and chemotherapy: a Childrens Cancer Group randomized trial. J Clin Oncol 13 (7): 1687-96, 1995.
-
Reddy AT, Janss AJ, Phillips PC, et al.: Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer 88 (9): 2189-93, 2000.
-
Timmermann B, Kortmann RD, Kühl J, et al.: Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol 20 (3): 842-9, 2002.
-
Jakacki RI, Burger PC, Kocak M, et al.: Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: a report from the Children's Oncology Group. Pediatr Blood Cancer 62 (5): 776-83, 2015.
-
Chintagumpala M, Hassall T, Palmer S, et al.: A pilot study of risk-adapted radiotherapy and chemotherapy in patients with supratentorial PNET. Neuro Oncol 11 (1): 33-40, 2009.
-
Johnston DL, Keene DL, Lafay-Cousin L, et al.: Supratentorial primitive neuroectodermal tumors: a Canadian pediatric brain tumor consortium report. J Neurooncol 86 (1): 101-8, 2008.
-
Geyer JR, Sposto R, Jennings M, et al.: Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol 23 (30): 7621-31, 2005.
-
Marec-Berard P, Jouvet A, Thiesse P, et al.: Supratentorial embryonal tumors in children under 5 years of age: an SFOP study of treatment with postoperative chemotherapy alone. Med Pediatr Oncol 38 (2): 83-90, 2002.
-
Grill J, Sainte-Rose C, Jouvet A, et al.: Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol 6 (8): 573-80, 2005.
-
Fangusaro J, Finlay J, Sposto R, et al.: Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer 50 (2): 312-8, 2008.
-
Friedrich C, von Bueren AO, von Hoff K, et al.: Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol 15 (2): 224-34, 2013.
Treatment of Newly Diagnosed MedulloepitheliomaThere are few data on which to base treatment of newly diagnosed medulloepithelioma and embryonal tumor with multilayered rosettes (ETMR). Treatment considerations are usually the same as those for children with high-risk medulloblastoma and for children aged 3 years and younger at diagnosis with other embryonal tumors. (Refer to the Children Older Than 3 Years with High-Risk Medulloblastoma and the Children Aged 3 Years and Younger sections of this summary for more information.) Prognosis is poor, with 5-year survival rates ranging between 0% and 30%.[1,2,3] Current Clinical Trials Check the list of NCI-supported cancer clinical trials that are now accepting patients with childhood medulloepithelioma. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. References:
-
Louis DN, Ohgaki H, Wiestler OD, et al.: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114 (2): 97-109, 2007.
-
Sharma MC, Mahapatra AK, Gaikwad S, et al.: Pigmented medulloepithelioma: report of a case and review of the literature. Childs Nerv Syst 14 (1-2): 74-8, 1998 Jan-Feb.
-
Müller K, Zwiener I, Welker H, et al.: Curative treatment for central nervous system medulloepithelioma despite residual disease after resection. Report of two cases treated according to the GPHO Protocol HIT 2000 and review of the literature. Strahlenther Onkol 187 (11): 757-62, 2011.
Treatment of Newly Diagnosed PineoblastomaChildren Older Than 3 Years Standard treatment options Standard treatment options for children older than 3 years with newly diagnosed pineoblastoma include the following: - Surgery.
- Adjuvant therapy.
- Radiation therapy.
- Chemotherapy.
Surgery Surgery is usually the initial treatment for pineoblastoma for diagnosis.[1] Total and near-total resection is infrequently obtained in pineoblastomas, and the impact of the degree of resection on outcome is unknown.[2,3] Adjuvant therapy The usual postsurgical treatment for pineoblastomas begins with radiation therapy, although some trials have utilized preradiation chemotherapy. The total dose of radiation therapy to the tumor site is 54 Gy to 55.8 Gy using conventional fractionation.[2,3] Adjuvant radiation therapy and chemotherapy - Craniospinal irradiation with doses ranging between 23.4 Gy and 36 Gy are also recommended because of the propensity of this tumor to disseminate throughout the subarachnoid space.[2,3]
- Chemotherapy is usually utilized in the same way as outlined for high-risk medulloblastomas in children with nondisseminated disease at the time of diagnosis.
- Five-year disease-free survival is more than 50% in children with localized disease at diagnosis undergoing aggressive resection.[2,3,4]
- For patients with disseminated disease at the time of diagnosis, survival is considerably poorer.[2,3]
Treatment options under clinical evaluation For patients with pineoblastoma, a variety of different treatment approaches are under evaluation, including the use of higher doses of chemotherapy after radiation therapy supported by peripheral stem cell rescue and the use of chemotherapy during radiation therapy. Children Aged 3 Years and Younger Biopsy is usually performed to diagnose pineoblastoma. Children aged 3 years and younger with pineoblastoma are usually treated initially with chemotherapy in the hope of delaying, if not obviating, the need for radiation therapy.[5] Overall prognosis for this group of children remains very poor. All five children younger than 3 years who were treated with chemotherapy on two sequential multicenter prospective clinical trials died.[6][Level of evidence: 2A] In children responding to chemotherapy, the timing and amount of radiation therapy required after chemotherapy is unclear. The addition of craniospinal irradiation to chemotherapy-based regimens may successfully treat some children but with anticipated neurodevelopmental decline.[7][Level of evidence: 2A] High-dose, marrow-ablative chemotherapy with autologous bone marrow rescue or peripheral stem cell rescue has been used with some success in young children.[8][Level of evidence: 2Di] Current Clinical Trials Check the list of NCI-supported cancer clinical trials that are now accepting patients with untreated childhood pineoblastoma. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. References:
-
Jakacki RI, Burger PC, Kocak M, et al.: Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: a report from the Children's Oncology Group. Pediatr Blood Cancer 62 (5): 776-83, 2015.
-
Jakacki RI, Zeltzer PM, Boyett JM, et al.: Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol 13 (6): 1377-83, 1995.
-
Timmermann B, Kortmann RD, Kühl J, et al.: Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol 20 (3): 842-9, 2002.
-
Gururangan S, McLaughlin C, Quinn J, et al.: High-dose chemotherapy with autologous stem-cell rescue in children and adults with newly diagnosed pineoblastomas. J Clin Oncol 21 (11): 2187-91, 2003.
-
Mason WP, Grovas A, Halpern S, et al.: Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol 16 (1): 210-21, 1998.
-
Hinkes BG, von Hoff K, Deinlein F, et al.: Childhood pineoblastoma: experiences from the prospective multicenter trials HIT-SKK87, HIT-SKK92 and HIT91. J Neurooncol 81 (2): 217-23, 2007.
-
Friedrich C, von Bueren AO, von Hoff K, et al.: Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol 15 (2): 224-34, 2013.
-
Fangusaro J, Finlay J, Sposto R, et al.: Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer 50 (2): 312-8, 2008.
Treatment of Recurrent Childhood CNS Embryonal TumorsRecurrence of all forms of central nervous system (CNS) embryonal tumors is not uncommon and usually occurs within 36 months of treatment. However, recurrent tumors may develop many years after initial treatment.[1,2] Disease may recur at the primary site or may be disseminated at the time of relapse. Sites of noncontiguous relapse may include the spinal leptomeninges, intracranial sites, and cerebrospinal fluid, in isolation or in any combination, and may be associated with primary tumor relapse.[1,2,3] Extraneural disease relapse may occur but is rare and is seen primarily in patients treated with radiation therapy alone.[4][Level of evidence: 3iiiA] Studies have found that even in patients with nondisseminated disease at diagnosis, and independent of the dose of radiation therapy or the type of chemotherapy, approximately one-third of patients will relapse at the primary tumor site alone; one-third will relapse at the primary tumor site plus distant sites; and one-third will relapse at distant sites without relapse at the primary site.[1,2,3] Treatment Options There are no standard treatment options for recurrent childhood CNS embryonal tumors. (Refer to the Treatment for Recurrent Childhood CNS Atypical Teratoid/Rhabdoid Tumor section in the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment for more information.) For most children, treatment is palliative and disease control is transient in patients previously treated with radiation therapy and chemotherapy, with more than 90% progressing within 12 to 18 months. For young children, predominantly those younger than 3 years at diagnosis who were never treated with radiation therapy, longer-term control with reoperation, radiation therapy, and chemotherapy is possible.[3,5,6,7] Treatment approaches may include the following: - Surgery.
- Radiation therapy.
- Chemotherapy.
- High-dose chemotherapy with stem cell rescue.
- Molecularly targeted therapy.
Surgery At the time of relapse, a complete evaluation for extent of recurrence is indicated for all embryonal tumors. Biopsy or surgical resection may be necessary for confirmation of relapse because other entities such as secondary tumors and treatment-related brain necrosis may be clinically indistinguishable from tumor recurrence. The need for surgical intervention must be individualized on the basis of the initial tumor type, the length of time between initial treatment and the reappearance of the lesion, and clinical symptomatology. Radiation therapy Patients with recurrent embryonal tumors who have already received radiation therapy and chemotherapy may be candidates for further radiation therapy depending on the site and dose of previous radiation, including reirradiation at the primary tumor site, focal areas of radiation therapy to sites of disseminated disease and, rarely, craniospinal radiation therapy.[8] In most cases, such therapy is palliative. Stereotactic radiation therapy and/or salvage chemotherapy can also be used (see below).[9] Chemotherapy - Recurrent CNS embryonal tumors can be responsive to chemotherapeutic agents used singularly or in combination, including cyclophosphamide, cisplatin, carboplatin, lomustine, etoposide, topotecan, temozolomide, and antiangiogenic metronomic therapy.[5,10,11,12,13,14,15,16,17,18]; [19,20][Level of evidence: 2A]
- Approximately 30% to 50% of these patients will have objective responses to conventional chemotherapy, but long-term disease control is rare.
- For select patients with recurrent medulloblastoma-primarily infants and young children who were treated at the time of diagnosis with chemotherapy alone and developed local recurrence-long-term disease control may be obtained after further treatment with chemotherapy plus local radiation therapy; this potential may be greatest in patients who are able to undergo complete resection of the recurrent disease.[21][Level of evidence: 2A]; [22][Level of evidence: 3iiiA]
High-dose chemotherapy with stem cell rescue For patients who have previously received radiation therapy, higher-dose chemotherapeutic regimens, supported with autologous bone marrow rescue or peripheral stem cell support, have been used with variable results.[6,7,23,24,25,26][Level of evidence: 2A]; [27][Level of evidence: 3iiB]; [28,29][Level of evidence: 3iiiA] - With such regimens, objective response is frequent, occurring in 50% to 75% of patients; however, long-term disease control is obtained in fewer than 30% of patients and is seen primarily in patients in first relapse and in those with only localized disease at the time of relapse.[7]; [26][Level of evidence: 2A]; [27][Level of evidence: 3iiB]
- Additionally, results from national trials for relapsed medulloblastoma that specified intent to transplant as part of their treatment plan showed that only approximately 5% of patients initiating retrieval therapy achieve long-term disease-free survival with this strategy.[26,30] Thus, studies that report from the time of transplant overestimate the benefit of transplant-based approaches for the total population of relapsing patients.
- Long-term disease control for patients with disseminated disease is infrequent.[31][Level of evidence: 3iA]
Molecularly targeted therapy With the increased knowledge of the molecular and genetic changes associated with different subtypes of medulloblastoma, molecularly targeted therapy, also called precision therapy, is being actively explored in children with recurrent disease. In patients with recurrent sonic hedgehog (SHH) subgroup medulloblastomas, the SHH PTCH1 inhibitor vismodegib demonstrated radiographic responses in 3 of 12 pediatric-aged patients, with two responses being sustained for less than 2 months and one response for more than 6 months. Only patients with upstream mutations of the SHH pathway, at the level of PTCH1 or SMO, responded.[32] Current Clinical Trials Check the list of NCI-supported cancer clinical trials that are now accepting patients with recurrent childhood pineoblastoma, recurrent childhood medulloblastoma and childhood medulloepithelioma. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. References:
-
Taylor RE, Bailey CC, Robinson K, et al.: Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 21 (8): 1581-91, 2003.
-
Packer RJ, Goldwein J, Nicholson HS, et al.: Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol 17 (7): 2127-36, 1999.
-
Oyharcabal-Bourden V, Kalifa C, Gentet JC, et al.: Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. J Clin Oncol 23 (21): 4726-34, 2005.
-
Mazloom A, Zangeneh AH, Paulino AC: Prognostic factors after extraneural metastasis of medulloblastoma. Int J Radiat Oncol Biol Phys 78 (1): 72-8, 2010.
-
Gentet JC, Doz F, Bouffet E, et al.: Carboplatin and VP 16 in medulloblastoma: a phase II Study of the French Society of Pediatric Oncology (SFOP). Med Pediatr Oncol 23 (5): 422-7, 1994.
-
Kadota RP, Mahoney DH, Doyle J, et al.: Dose intensive melphalan and cyclophosphamide with autologous hematopoietic stem cells for recurrent medulloblastoma or germinoma. Pediatr Blood Cancer 51 (5): 675-8, 2008.
-
Butturini AM, Jacob M, Aguajo J, et al.: High-dose chemotherapy and autologous hematopoietic progenitor cell rescue in children with recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors: the impact of prior radiotherapy on outcome. Cancer 115 (13): 2956-63, 2009.
-
Wetmore C, Herington D, Lin T, et al.: Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer 120 (23): 3731-7, 2014.
-
Abe M, Tokumaru S, Tabuchi K, et al.: Stereotactic radiation therapy with chemotherapy in the management of recurrent medulloblastomas. Pediatr Neurosurg 42 (2): 81-8, 2006.
-
Friedman HS, Oakes WJ: The chemotherapy of posterior fossa tumors in childhood. J Neurooncol 5 (3): 217-29, 1987.
-
Needle MN, Molloy PT, Geyer JR, et al.: Phase II study of daily oral etoposide in children with recurrent brain tumors and other solid tumors. Med Pediatr Oncol 29 (1): 28-32, 1997.
-
Gaynon PS, Ettinger LJ, Baum ES, et al.: Carboplatin in childhood brain tumors. A Children's Cancer Study Group Phase II trial. Cancer 66 (12): 2465-9, 1990.
-
Allen JC, Walker R, Luks E, et al.: Carboplatin and recurrent childhood brain tumors. J Clin Oncol 5 (3): 459-63, 1987.
-
Ashley DM, Longee D, Tien R, et al.: Treatment of patients with pineoblastoma with high dose cyclophosphamide. Med Pediatr Oncol 26 (6): 387-92, 1996.
-
Lefkowitz IB, Packer RJ, Siegel KR, et al.: Results of treatment of children with recurrent medulloblastoma/primitive neuroectodermal tumors with lomustine, cisplatin, and vincristine. Cancer 65 (3): 412-7, 1990.
-
Friedman HS, Mahaley MS Jr, Schold SC Jr, et al.: Efficacy of vincristine and cyclophosphamide in the therapy of recurrent medulloblastoma. Neurosurgery 18 (3): 335-40, 1986.
-
Castello MA, Clerico A, Deb G, et al.: High-dose carboplatin in combination with etoposide (JET regimen) for childhood brain tumors. Am J Pediatr Hematol Oncol 12 (3): 297-300, 1990.
-
Cefalo G, Massimino M, Ruggiero A, et al.: Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi-institutional phase II trial. Neuro Oncol 16 (5): 748-53, 2014.
-
Minturn JE, Janss AJ, Fisher PG, et al.: A phase II study of metronomic oral topotecan for recurrent childhood brain tumors. Pediatr Blood Cancer 56 (1): 39-44, 2011.
-
Peyrl A, Chocholous M, Kieran MW, et al.: Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer 59 (3): 511-7, 2012.
-
Ridola V, Grill J, Doz F, et al.: High-dose chemotherapy with autologous stem cell rescue followed by posterior fossa irradiation for local medulloblastoma recurrence or progression after conventional chemotherapy. Cancer 110 (1): 156-63, 2007.
-
Bakst RL, Dunkel IJ, Gilheeney S, et al.: Reirradiation for recurrent medulloblastoma. Cancer 117 (21): 4977-82, 2011.
-
Dunkel IJ, Boyett JM, Yates A, et al.: High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children's Cancer Group. J Clin Oncol 16 (1): 222-8, 1998.
-
Park JE, Kang J, Yoo KH, et al.: Efficacy of high-dose chemotherapy and autologous stem cell transplantation in patients with relapsed medulloblastoma: a report on the Korean Society for Pediatric Neuro-Oncology (KSPNO)-S-053 study. J Korean Med Sci 25 (8): 1160-6, 2010.
-
Gilman AL, Jacobsen C, Bunin N, et al.: Phase I study of tandem high-dose chemotherapy with autologous peripheral blood stem cell rescue for children with recurrent brain tumors: a Pediatric Blood and MarrowTransplant Consortium study. Pediatr Blood Cancer 57 (3): 506-13, 2011.
-
Pizer B, Donachie PH, Robinson K, et al.: Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: results of a Children's Cancer and Leukaemia Group study. Eur J Cancer 47 (9): 1389-97, 2011.
-
Massimino M, Gandola L, Spreafico F, et al.: No salvage using high-dose chemotherapy plus/minus reirradiation for relapsing previously irradiated medulloblastoma. Int J Radiat Oncol Biol Phys 73 (5): 1358-63, 2009.
-
Gururangan S, Krauser J, Watral MA, et al.: Efficacy of high-dose chemotherapy or standard salvage therapy in patients with recurrent medulloblastoma. Neuro Oncol 10 (5): 745-51, 2008.
-
Dunkel IJ, Gardner SL, Garvin JH Jr, et al.: High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol 12 (3): 297-303, 2010.
-
Gajjar A, Pizer B: Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer 54 (4): 649-51, 2010.
-
Bowers DC, Gargan L, Weprin BE, et al.: Impact of site of tumor recurrence upon survival for children with recurrent or progressive medulloblastoma. J Neurosurg 107 (1 Suppl): 5-10, 2007.
-
Robinson GW, Orr BA, Wu G, et al.: Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J Clin Oncol 33 (24): 2646-54, 2015.
Changes to This Summary (01 / 27 / 2017)The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. This summary was extensively revised. This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages. About This PDQ SummaryPurpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood central nervous system embryonal tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: - be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Childhood Central Nervous System Embryonal Tumors Treatment are: - Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Roger J. Packer, MD (Children's National Health System)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Central Nervous System Embryonal Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-cns-embryonal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389418] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2017-01-27 Last modified on: 8 September 2017
|
|