Childhood Hematopoietic Cell Transplantation (PDQ®): Treatment - Health Professional Information [NCI]
Childhood Hematopoietic Cell Transplantation (PDQ®): Treatment - Health Professional Information [NCI]Skip to the navigationGeneral Information About Hematopoietic Cell Transplantation (HCT)Rationale for HCT Blood and marrow transplantation (BMT) or HCT is a procedure that involves infusion of hematopoietic stem cells (hematopoietic progenitor cells) to reconstitute the hematopoietic system of a patient. The infusion of hematopoietic stem cells generally follows a preparative regimen given to the patient consisting of agents designed to do the following: - Create marrow space.
- Suppress the patient's immune system to prevent rejection.
- Intensively treat malignant cells in patients with cancer.
HCT is currently used in the following three clinical scenarios: - Treatment of malignancies.
- Replacement or modulation of an absent or poorly functioning hematopoietic or immune system.
- Treatment of genetic diseases in which an insufficient expression of the affected gene product by the patient can be partially or completely overcome by circulating hematopoietic cells transplanted from a donor with normal gene expression.
Autologous Versus Allogeneic HCT The two major transplant approaches currently in use are the following: - Autologous (using the patient's own hematopoietic stem cells).
- Allogeneic (using related or unrelated donor hematopoietic stem cells).
Autologous transplant treats cancer by exposing patients to mega-dose (myeloablative) therapy with the intent of overcoming chemotherapy resistance in tumor cells, followed by infusion of the patient's previously stored hematopoietic stem cells. It has also been used to attempt to reset the immune system in severe autoimmune disorders. For autologous transplant to work, the following must apply: - The higher chemotherapy/radiation therapy dose that can be used because of hematopoietic stem cell support achieves a significantly higher cell kill of the disease. This may include increased tumor kill in areas where standard-dose chemotherapy has less penetration (central nervous system).
- Meaningful percentages of cure or long-term remission from the disease must occur without significant nonhematopoietic toxicities that would otherwise limit the therapeutic benefit achieved.
Current pediatric indications for autologous transplant include patients with certain lymphomas, neuroblastoma, and brain tumors. Allogeneic transplant approaches to cancer treatment also may involve high-dose therapy, but because of immunologic differences between the donor and recipient, an additional graft-versus-tumor (GVT) or graft-versus-leukemia (GVL) treatment effect can occur. Although autologous approaches are associated with less short-term mortality, many malignancies are resistant to mega-dose therapy alone and/or involve the bone marrow, thus requiring allogeneic approaches for optimal outcome. Determining When HCT Is Indicated: Comparison of HCT and Chemotherapy Outcomes Because the outcomes using chemotherapy and HCT treatments have been changing over time, regular comparisons between these approaches should be performed to continually redefine optimal therapy for a given patient. For some diseases, randomized trials or intent-to-treat using a HLA-matched sibling donor have established the benefit of HCT by direct comparison.[1,2] However, for very high-risk patients such as those with early relapse of acute lymphoblastic leukemia (ALL), randomized trials have not been feasible because of investigator bias.[3,4] In general, HCT typically offers benefit only to children at high risk of relapse with standard chemotherapy approaches. Accordingly, treatment schemas that accurately identify these high-risk patients and offer HCT if reasonably HLA-matched donors are available have come to be the preferred approach for many diseases.[5] Less well-established, higher-risk approaches to HCT are generally reserved for only the very highest-risk patients. However, higher-risk approaches such as haploidentical transplantation are becoming safer and more efficacious and are increasingly being used interchangeably with fully matched allogeneic approaches.[6,7,8,9] (Refer to the Haploidentical HCT section of this summary for more information.) When comparisons of similar patients treated with HCT or chemotherapy are made in the setting where randomized or intent-to-treat studies are not feasible, the following issues should be considered: - Remission/disease status: Comparisons between HCT and chemotherapy should include only patients who obtain remission, preferably after similar approaches to salvage therapy, because patients failing to obtain remission do very poorly with any therapy. To account for time-to-transplant bias, the chemotherapy comparator arm should include only patients who maintained remission until the median time to HCT. The HCT comparator arm should also include only patients who achieved the initial remission mentioned above and maintained that remission until the time of HCT. High-risk and intermediate-risk patient groups should not be combined because a benefit for HCT in the high-risk group can be masked when outcomes are similar to those achieved in the intermediate-risk group.[10]
- Therapy approaches used for comparison: Comparisons should be made with the best or most commonly used chemotherapy and HCT approaches utilized during the time frame under study.
- HCT approach: HCT approaches that are very high risk or have documented lower rates of survival should not be combined for analysis with standard-risk HCT approaches.
- Criteria for relapse: Risk factors for relapse should be carefully defined, and analysis should be based on the most current knowledge of risk.
- Selection bias: Attempts should be made to understand and eliminate or correct for selection bias. Examples include the following:
- Higher -risk patients preferentially undergoing HCT (i.e., patients who take several rounds to achieve remission or who relapse after obtaining remission and go back into a subsequent remission prior to HCT).
- Sicker patients deferred from HCT because of comorbidities.
- Patient or parent refusal.
- Lack of or inability to obtain insurance approval for HCT.
- Lack of access to HCT because of distance or inability to travel.
- Related to the time-to-transplant bias noted above, patients who undergo HCT after relapse or recurrence are a subset of all patients with a disease recurrence and will be selected from those who are able to obtain a remission and remain healthy enough to undergo HCT.
One source of bias difficult to control for or detect is physician bias for or against HCT. The effect of access to HCT and therapeutic bias on outcomes of pediatric malignancies where HCT may be indicated has been poorly studied to date. References:
-
Matthay KK, Villablanca JG, Seeger RC, et al.: Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med 341 (16): 1165-73, 1999.
-
Woods WG, Neudorf S, Gold S, et al.: A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood 97 (1): 56-62, 2001.
-
Lawson SE, Harrison G, Richards S, et al.: The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol 108 (3): 531-43, 2000.
-
Gaynon PS, Harris RE, Altman AJ, et al.: Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol 24 (19): 3150-6, 2006.
-
Schrauder A, von Stackelberg A, Schrappe M, et al.: Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant 41 (Suppl 2): S71-4, 2008.
-
Bertaina A, Merli P, Rutella S, et al.: HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 124 (5): 822-6, 2014.
-
Handgretinger R, Chen X, Pfeiffer M, et al.: Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci 1106: 279-89, 2007.
-
Huang XJ, Liu DH, Liu KY, et al.: Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38 (4): 291-7, 2006.
-
Luznik L, Fuchs EJ: High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 47 (1-3): 65-77, 2010.
-
Pulsipher MA, Peters C, Pui CH: High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant 17 (1 Suppl): S137-48, 2011.
Autologous HCTCollection and Storage of Autologous Hematopoietic Stem Cells Autologous procedures require collection of growth-factor-mobilized peripheral blood stem cells (PBSCs) from patients by the process of apheresis. Bone marrow can be used for the transplant, but PBSCs have been shown to lead to quicker blood count recovery and less toxicity. Patients under consideration for autologous hematopoietic cell transplantation (HCT) are generally given chemotherapy to determine tumor responsiveness and minimize risk of tumor contamination in their bone marrow. After a number of rounds of chemotherapy, they undergo the apheresis procedure, either as their blood counts recover from chemotherapy or during a break between chemotherapy treatments. Growth factors such as granulocyte colony-stimulating factor (G-CSF) are used to increase the number of circulating stem and progenitor cells (CD34+ cells). Collection centers monitor the CD34-positive number in the patient and product each day to determine the best time to begin collection and when collection is complete. Patients with poorly mobilized CD34-positive cells can often have their cells successfully collected using alternative mobilization approaches (e.g., plerixafor).[1] The collected PBSCs are cryopreserved for later use. After completion of an intensive preparative regimen using high-dose chemotherapy, which varies according to tumor, the PBSCs are administered back to the patient at the time of transplant. General Indications for Autologous Procedures/Preparative Regimens/Tumor Purging In pediatrics, the most common autologous transplant indications are the following: - High-risk neuroblastoma. (Refer to the PDQ summary on Neuroblastoma Treatment for more information.)
- Relapsed Hodgkin lymphoma and non-Hodgkin lymphoma. (Refer to the PDQ summaries on Childhood Hodgkin Lymphoma Treatment and Childhood Non-Hodgkin Lymphoma Treatment for more information.)
- High-risk and relapsed brain tumors.
- Relapsed or resistant germ cell tumors. (Refer to the PDQ summaries on Childhood Central Nervous System Germ Cell Tumors Treatment and Childhood Extracranial Germ Cell Tumors Treatment for more information.)
Tumor-specific regimens are described in disease-specific PDQ treatment summaries. Preparative regimens for allogeneic transplant are needed mainly to ensure engraftment of the donor marrow or cord blood. Use of high-dose tumor-specific agents, however, has not shown benefit, especially if such agents add toxicity to the approach. Unlike allogeneic procedures, the tumor-specific activity and intensity of agents used for autologous regimens have been shown to be important in improving survival. One concern with autologous approaches for these and other tumor types has been the contamination of the collected stem cell product by persistent tumor cells. Although many techniques have been developed to remove or purge tumor cells from products, most studies looking into these approaches have shown no benefit to tumor purging.[2] References:
-
Patel B, Pearson H, Zacharoulis S: Mobilisation of haematopoietic stem cells in paediatric patients, prior to autologous transplantation following administration of plerixafor and G-CSF. Pediatr Blood Cancer 62 (8): 1477-80, 2015.
-
Kreissman SG, Villablanca JG, Seeger RC, et al.: A randomized phase III trial of myeloablative autologous peripheral blood stem cell (PBSC) transplant (ASCT) for high-risk neuroblastoma (HR-NB) employing immunomagnetic purged (P) versus unpurged (UP) PBSC: A Children's Oncology Group study. [Abstract] J Clin Oncol 26 (Suppl 15): A-10011, 2008. Also available online. Last accessed September 23, 2016.
Allogeneic HCTImproved Outcomes After Allogeneic Transplantation Over the past one to two decades, significant advances have led to improved outcomes after allogeneic hematopoietic cell transplantation (HCT).[1,2,3] The most significant improvements in survival occurred in unrelated and alternative donor procedures.[4,5,6] Possible explanations for these improvements in survival include improved patient selection, better supportive care, refined treatment regimens, improved approaches specific to stem cell sources, and better human leukocyte antigen (HLA) typing. All of these factors may have contributed to better outcomes; however, the section below focuses on modifiable aspects of HCT (i.e., optimization of HLA typing and selection of stem cell sources). HLA Matching and Hematopoietic Stem Cell Sources HLA overview Appropriate matching between donor and recipient HLA in the major histocompatibility complex located on chromosome 6 is essential to successful allogeneic HCT (refer to Table 1).
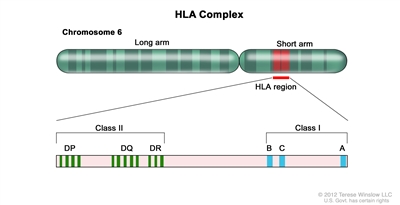
Figure 1. HLA Complex. Human chromosome 6 with amplification of the HLA region. The locations of specific HLA loci for the class I B, C, and A alleles and the class II DP, DQ, and DR alleles are shown.
HLA class I (A, B, C, etc.) and class II (DRB1, DQB1, DPB1, etc.) alleles are highly polymorphic; therefore, finding appropriately matched unrelated donors is a challenge for some patients, especially those of certain racial groups (e.g., African Americans and Hispanics).[7,8] Because full siblings of cancer patients have a 25% chance of being HLA matched, they have been the preferred source of allogeneic hematopoietic stem cells. Early serologic techniques of HLA assessment defined a number of HLA antigens, but more precise DNA methodologies have shown HLA allele-level mismatches in up to 40% of serologic HLA antigen matches. These differences are clinically relevant because use of donors with allele-level mismatches affects survival and rates of graft-versus-host disease (GVHD) to a degree similar to that in patients with antigen-level mismatches.[9] Because of this, DNA-based allele-level HLA typing is standard when unrelated donors are being chosen. Table 1. Level of HLA Typing Currently Used for Different Hematopoietic Stem Cell Sourcesa,b,c | Class I Antigens | Class II Antigens |
|---|
| BM = bone marrow; PBSC = peripheral blood stem cells. | | a HLA antigen: A serologically defined, low-resolution method of defining an HLA protein. Differs from allele-level typing half of the time. Designated by the first two numbers (i.e., HLA B 35:01-antigen is HLA B 35). | | b HLA allele: A higher resolution method of defining unique HLA proteins by typing their gene through sequencing or other DNA-based methods that detect unique differences. Designated by at least four numbers (i.e., HLA B 35:01). | | c Consensus recommendations for HLA typing, including extended class II typing of mismatched donors, have been published from the National Cancer Institute/National Heart, Lung, and Blood Institute-sponsored Blood and Marrow Transplant Clinical Trials Network.[10] | | d Siblings need confirmation that they have fully matched haplotypes with no crossovers in the A to DRB1 region. If parental typing is performed and haplotypes are established, antigen-level typing of class I is adequate. With no parental haplotypes, allele-level typing of eight alleles is recommended. | | e Parent, cousin, etc., with a phenotypic match or near-complete HLA match. | | Stem Cell Source | HLA A | HLA B | HLA C | HLA DRB1 | HLA DQB1;HLA DPB1; HLA DR3,4,5 | | Matched siblingd BM/PBSCs | Antigen or allele | Antigen or allele | Optional | Allele | | | Mismatched sibling/other related donore BM/PBSCs | Allele | Allele | Allele | Allele | Recommended, if mismatches are present | | Unrelated donor BM/PBSCs | Allele | Allele | Allele | Allele | Recommended, if mismatches are present | | Unrelated donor cord blood | Antigen (allele recommended ) | Antigen (allele recommended ) | Allele recommended | Allele | | Table 2. Definitions of the Numbers Describing HLA Antigens and Alleles Matching| If These HLA Antigens and Alleles Match: | Then the Donor Is Considered to be This Type of Match: |
|---|
| A, B, and DRB1 | 6/6 | | A, B, C, and DRB1 | 8/8 | | A, B, C, DRB1, and DQB1 | 10/10 | | A, B, C, DRB1, DQB1, and DPB1 | 12/12 | HLA matching considerations for sibling and related donors The most commonly used related donor is a sibling from the same parents who is HLA-matched for HLA A, HLA B, and HLA DRB1 at a minimum, at the antigen level. Given the distance on chromosome 6 between HLA A and HLA DRB1, there is approximately a 1% possibility of a crossover event occurring in a possible sibling match. Because a crossover event could involve the HLA C antigen and because parents may share HLA antigens that actually differ at the allele level, many centers perform allele-level typing of possible sibling donors at all of the key HLA antigens (HLA A, B, C, and DRB1). Any related donor that is not a full sibling should have full HLA typing because similar haplotypes from different parents could differ at the allele level. Although single-antigen mismatched related donors (5/6 antigen matched) have been used interchangeably with matched sibling donors in some studies, a large Center for International Blood and Marrow Transplant Research (CIBMTR) study in pediatric HCT recipients showed that use of 5/6 antigen matched related donors who are not siblings results in rates of GVHD and overall survival (OS) equivalent to rates in 8/8 allele level matched unrelated donors and slightly inferior survival than in fully matched siblings.[11] HLA matching considerations for unrelated donors Optimal outcomes are achieved in unrelated allogeneic marrow transplantation when the pairs of antigens at HLA A, B, C, and DRB1 are matched between the donor and the recipient at the allele level (termed an 8/8 match).[12] A single antigen/allele mismatch at any of these antigens (7/8 match) lowers the probability of survival between 5% and 10%, with a similar increase in the amount of significant (grades III-IV) acute GVHD.[12] Of these four antigen pairs, different reports have shown HLA A, C, and DRB1 mismatches to potentially be more highly associated with mortality than the other antigens,[9,12,13] but the differences in outcome are small and inconsistent, making it very difficult to conclude presently that one can pick a more favorable mismatch by choosing one type of antigen mismatch above another. Many groups are attempting to define specific antigens or pairs of antigens that are associated with either good or poor outcomes. A specific HLA C mismatch (HLA-C*03:03/03:04) has outcomes similar to a match; therefore, selection of this mismatch is desirable in an otherwise matched donor/pair combination.[14] It is well understood that class II antigen DRB1 mismatches increase GVHD and worsen survival.[13] Subsequent data have also shown that multiple mismatches of DQB1, DPB1, and DR3,4,5 lead to worse outcomes in the setting of less than 8/8 matches.[15] DPB1 mismatches have been extensively studied and classified as permissive or nonpermissive based on T-cell epitope matching. Patients with 10/10 matches and nonpermissive DPB1 mismatches have more transplant-related mortality but have survival rates similar to those with DPB1 matches or permissive matches. Those with 9/10 matches who have nonpermissive DPB1 mismatches had worse survival than did those with permissive mismatches or DPB1 matches.[16,17,18] With these findings in mind, although a 7/8 or 8/8 matched unrelated donor can be used routinely, centers may be able to further improve outcomes by the use of extended typing of DQB1, DPB1, and DR3,4,5, especially in the context of a less-than-8/8-matched donor.[16,17,18] In addition, extended HLA testing can also allow the selection of appropriate donors in the context of HLA-sensitized patients to avoid the potential risk of graft failure.[19,20] HLA sensitization is detected by testing for the presence of specific anti-HLA antibodies and avoidance of donors who have any HLA antigens associated with the antibodies present in the recipient. Finally, the use of younger donors and blood type-compatible unrelated donors may further improve outcomes.[10]
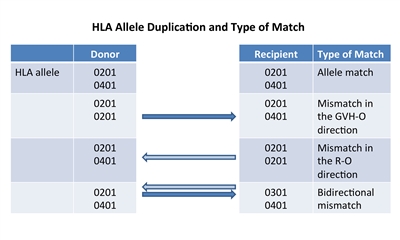
Figure 2. HLA allele duplication in a donor or recipient results in a half match and a mismatch that will either occur in a direction that promotes GVHD (GVH-O) or a direction that promotes rejection (R-O).
If a donor or recipient has a duplication of one of their HLA alleles, they will have a half match and a mismatch only in one direction. Figure 2 illustrates that these mismatches will occur in either a direction that promotes GVHD (GVH-O) or a direction that promotes rejection (R-O). When 8/8 matched unrelated donors are compared with 7/8 donors mismatched in the GVH-O direction, 7/8 mismatched in the R-O direction, or 7/8 mismatched in both directions, the mismatch in the R-O direction leads to rates of grades III and IV acute GVHD similar to rates in the 8/8 matched and better than in the other two combinations. The 7/8 mismatched in only the R-O direction is preferred over GVH-O and bidirectional mismatches.[21] It is important to note that this observation in unrelated donors differs from observations in cord blood recipients outlined below. HLA matching and cell dose considerations for unrelated cord blood HCT Another commonly used hematopoietic stem cell source is that of unrelated umbilical cord blood, which is harvested from donor placentas moments after birth. The cord blood is processed, cryopreserved, HLA typed, and banked. Unrelated cord blood transplantation has been successful with less-stringent HLA matching requirements compared with standard related or unrelated donors, probably because of limited antigen exposure experienced in utero and different immunological composition. Cord blood matching has traditionally been performed at an intermediate level for HLA A and B and at an allele level (high resolution) for DRB1. This means that attempted matching of only six antigens is necessary to choose units for transplantation. Although better outcomes occur when 6/6 or 5/6 HLA matched units are used,[22] successful HCT has occurred even with 4/6 or less matched units in many patients. In a large CIBMTR/Eurocord study, better matching at the allele level using eight antigens (matching for HLA A, B, C, and DRB1) resulted in less transplant-related mortality and improved survival. Best outcome was noted with 8/8 allele matching versus 4/8 to 7/8 matches, with poor survival in patients with five or more allele mismatches. Patients receiving 8/8 matched cord blood did not require higher cell doses for better outcomes; however, those with one to three allele mismatches had less transplant-related mortality with total nucleated cell counts greater than 3 × 107 /kg, and those with four allele mismatches required a total nucleated cell count greater than 5 × 107 /kg to decrease transplant-related mortality.[23] Many centers will type additional alleles and use the best match possible, but the impact of DQB1, DPB1, and DR3,4,5 mismatches has not been studied in detail. As in unrelated peripheral blood stem cells (PBSC) or bone marrow donors, extended HLA testing can support the selection of appropriate cord blood units in HLA-sensitized patients to avoid the potential risk of graft failure.[24,25] Evidence also suggests that selecting a mismatched cord blood unit, where the mismatch involves a noninherited maternal antigen, may improve survival.[26,27] As with unrelated donors, individuals can occasionally have duplicate HLA antigens (e.g., the HLA A antigen is 01 on both chromosomes). When this occurs in a donor product and the antigen is matched to one of the recipient antigens, the recipient immune response will see the donor antigens as matched (matched, in the rejection direction), but the donor immune response will see a mismatch in the recipient (mismatch in the GVHD direction). This variation of partial mismatching has been shown to be important in cord blood transplant outcomes. Mismatches that are only in the GVHD direction (GVH-O) lead to lower transplant-related mortality and overall mortality than in those with recipient direction only (R-O) mismatches.[28] R-O mismatches have outcomes similar to those caused by bidirectional mismatches.[29] Although some recommend using unidirectional mismatching as a criteria for cord blood selection, a Eurocord-EBMT analysis disputes the value of this type of mismatching.[30] Two aspects of umbilical cord blood HCT have made the practice more widely applicable. First, because a successful procedure can occur with multiple HLA mismatches, more than 95% of patients from a wide variety of ethnicities are able to find at least a 4/6 matched cord blood unit.[7,31] Second, as mentioned above, adequate cell dose (minimum 2-3 × 107 total nucleated cells/kg and 1.7 × 105 CD34+ cells/kg) has been shown to be associated with improved survival.[32,33] Total nucleated cells are generally used to judge units because techniques to measure CD34-positive doses have not been standardized. Because even large single umbilical cord blood units are only able to supply these minimum doses to recipients weighing up to 40 kg to 50 kg, early umbilical cord blood HCT focused mainly on smaller children. Later studies showed that this size barrier could be overcome by using two umbilical cord blood units, as long as each of the units is at least a 4/6 HLA match with the recipient; because two cords provide higher cell doses, umbilical cord blood transplantation is now used widely for larger children and adults.[34] If a single unit provides an adequate cell dose, there may be disadvantages to adding a second unit.[35][Level of evidence: 1iiA] Two randomized trials showed that in children who had adequately sized single units, the addition of a second unit did not alter relapse, transplant-related mortality, or survival rates, but was associated with higher rates of extensive chronic GVHD.[35,36] Comparison of stem cell products Currently, the following three stem cell products are used from both related and unrelated donors: - Bone marrow.
- PBSCs.
- Cord blood.
In addition, bone marrow or PBSCs can be T-cell depleted by several methods, and the resultant stem cell product has very different properties. Finally, partially HLA-matched (half or more antigens [haploidentical]) related bone marrow or PBSCs can be used after in vitro or in vivo T-cell depletion, and this product also behaves differently from other stem cell products. A comparison of stem cell products is presented in Table 3. Table 3. Comparison of Hematopoietic Stem Cell Products | PBSCs | BM | Cord Blood | T-cell-Depleted BM/PBSCs | Haploidentical T-cell-Depleted BM/PBSCs |
|---|
| BM = bone marrow; EBV-LPD = Epstein-Barr virus-associated lymphoproliferative disorder; GVHD = graft-versus-host disease; HCT = hematopoietic cell transplantation; PBSCs = peripheral blood stem cells. | | a Assuming no development of GVHD. If patients develop GVHD, immune reconstitution is delayed until resolution of the GVHD and discontinuation of immune suppression. | | b If a haploidentical donor is used, longer times to immune reconstitution may occur. | | T-cell content | High | Moderate | Low | Very low | Very low | | CD34+ content | Moderate-high | Moderate | Low (but higher potency) | Moderate-high | Moderate-high | | Time to neutrophil recovery | Rapid: median, 16 d (11-29 d)[37] | Moderate: median, 21 d (12-35 d)[37] | Slower: median, 23 d (11-133 d)[36] | Rapid: median, 16 d (9-40 d)[38] | Rapid: median, 13 d (10-20 d)[39] | | Early post-HCT risk of infections, EBV-LPD | Low-moderate | Moderate | High | Very High | Very High | | Risk of graft rejection | Low | Low-moderate | Moderate-high | Moderate-high | Moderate-high | | Time to immune reconstitutiona | Rapid (6-12 mo) | Moderate (6-18 mo) | Slow (6-24 mo) | Slow (6-24 mo) | Slow (9-24 mo)b | | Risk of acute GVHD | Moderate | Moderate | Moderate | Low | Low | | Risk of chronic GVHD | High | Moderate | Low | Low | Low | The main differences between the products are associated with the numbers of T cells and CD34-positive progenitor cells present; very high levels of T cells are present in PBSCs, intermediate numbers in bone marrow, and low numbers in cord blood and T-cell-depleted products. Patients receiving T-cell-depleted products or cord blood generally have slower hematopoietic recovery, increased risk of infection, late immune reconstitution, higher risks of nonengraftment, and increased risk of Epstein-Barr virus (EBV)-associated lymphoproliferative disorder. This is countered by lower rates of GVHD and an ability to offer transplantation to patients where full HLA matching is not available. Higher doses of T and other cells in PBSCs result in rapid neutrophil recovery and immune reconstitution, but also increased rates of chronic GVHD. There are only a few studies directly comparing outcomes of different stem cell sources/products in pediatric patients. The following results have been observed: - A retrospective registry study of pediatric patients who underwent HCT for acute leukemia compared those who received related donor bone marrow with those who received related donor PBSCs. Although the bone marrow and PBSC recipient cohorts differed some in their risk profiles, after statistical correction, increased risk of GVHD and transplant-related mortality associated with PBSC led to poorer survival in the PBSC group.[40]
- A retrospective study of Japanese children with acute leukemia compared 90 children who received PBSCs with 571 children who received bone marrow; the study confirmed higher transplant-related mortality due to GVHD and inferior survival among the children who received PBSCs.[41]
These reports, combined with a lack of prospective studies comparing bone marrow and PBSCs have led most pediatric transplant protocols to prefer bone marrow over PBSCs from related donors. For those requiring unrelated donors, a large Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trial that included a few pediatric patients randomly assigned patients to receive either bone marrow or PBSCs. This trial demonstrated that OS was identical using either source, but rates of chronic GVHD were significantly higher in the PBSC arm.[42] Published studies that compared unrelated cord blood and bone marrow have been retrospective, with weaknesses inherent in such analyses. In one study, pediatric patients with acute lymphoblastic leukemia (ALL) who underwent HCT and received 8/8 HLA allele-matched unrelated donor bone marrow were compared with those who received unrelated cord blood.[22] The analysis showed that the best survival occurred in recipients of 6/6 HLA-matched cord blood; survival after 8/8 HLA-matched unrelated bone marrow was slightly less and was statistically identical to survival for patients receiving 5/6 and 4/6 HLA-matched cord blood units. In a second study from a single center consisting of mostly adult patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and ALL, outcomes for cord blood recipients were compared with outcomes for recipients of matched and mismatched unrelated donor bone marrow/PBSCs. Better survival because of less relapse was noted in cord blood recipients, mainly resulting from superior survival in patients with minimal residual disease (MRD) present just before transplant. No difference was seen in relapse and survival between patients with pre-HCT MRD and patients without pre-HCT MRD if they received cord blood.[43] This result is controversial because it contradicts many other studies that showed that the presence of pre-HCT MRD in cord blood recipients leads to increased relapse and inferior survival.[44,45,46,47] On the basis of these studies, most transplant centers consider matched sibling bone marrow to be the preferred stem cell source/product. If a sibling donor is not available, fully matched unrelated donor bone marrow or PBSCs or HLA-matched (4/6 to 6/6) cord blood leads to similar survival. Although adult studies of T-cell-depleted unrelated bone marrow or PBSCs have shown outcomes similar to non-T-cell-depleted approaches, large pediatric trials or retrospective studies comparing T-cell-depleted matched or haploidentical bone marrow or PBSCs have not been conducted. Haploidentical HCT Early HCT studies demonstrated progressively higher percentages of patients experiencing severe GVHD and lower survival as the number of donor/recipient HLA mismatches increased.[48] Studies have further demonstrated that even with very high numbers of donors in unrelated donor registries, patients with rare HLA haplotypes and patients with certain ethnic backgrounds (e.g., Hispanic, African American, Asian-Pacific Islander, etc.) have a low chance of achieving desired levels of HLA matching (7/8 or 8/8 match at the allele level).[8] To allow access to HCT for patients without HLA-matched donor options, investigators developed techniques allowing use of siblings, parents, or other relatives who share only a single haplotype of the HLA complex with the patient and are thus half matches. Most approaches developed to date rely on intense T-cell depletion of the product before infusion into the patient. The main challenge associated with this approach is intense immune suppression with delayed immune recovery, which can result in lethal infections,[49] increased risk of EBV-lymphoproliferative disorder, and high rates of relapse.[50] This has generally led to inferior survival compared with matched HCT and has resulted in the procedure being generally practiced only at larger academic centers with a specific research focus on studying and developing this approach. Current approaches are rapidly evolving, as evidenced by the following, resulting in improved outcome, with some pediatric groups reporting survival similar to that of standard approaches.[51,52] - Newer techniques of T-cell depletion and add-back of specific cell populations (e.g., CD3 or alpha-beta CD3/CD19-negative selection) may decrease transplant-related mortality.[39,53]
- Reduced toxicity regimens have led to improved survival.
- Better supportive care has decreased the chance of morbidity from infection or EBV-lymphoproliferative disorder.[54]
- Some patient-donor combinations that have specific killer immunoglobulin-like receptor mismatches have shown decreased likelihood of relapse (refer to the Role of killer immunoglobulin-like receptor mismatching in HCT section of this summary for more information).
- Certain techniques, such as using combinations of granulocyte-colony stimulating factor (G-CSF)-primed bone marrow and PBSCs with posttransplant antibody-based T-cell depletion [55] or post-HCT cyclophosphamide (chemotherapeutic T-cell depletion),[56] have made these procedures more accessible to centers because expensive and complicated processing necessary for traditional T-cell depletion are not used.
Reported survival using many different types of haploidentical approaches varies between 25% and 80%, depending on the technique used and the risk of the patient undergoing the procedure.[50,51,55,56] Whether haploidentical approaches are superior to cord blood or other stem cell sources for a given patient group has not been determined because comparative studies have yet to be performed.[50] Other donor characteristics associated with outcome Although HLA matching has consistently been the most important factor associated with improved survival in nonhaploidentical allogeneic HCT, a number of other characteristics of the donor have been shown in studies to affect key outcomes. Higher cell dose from the donor (refer to the HLA matching and cell dose considerations for unrelated cord blood HCT section of this summary for more information) has also been shown to be important when related, unrelated, or haploidentical bone marrow or PBSC donors are used.[57,58] The effects of donor age, blood type, cytomegalovirus (CMV) status, gender, and parity of female donors have also been studied. Ideally, transplant centers should select donors based on the following characteristics: - Donor age. The youngest donor available is preferred.[59,60]
- Matched donor blood type.[61,62,63]
- CMV status of the recipient. CMV-negative donor matched to CMV-negative recipient and CMV-positive donor matched to CMV-positive recipient.[64]
- Donor sex and parity of female donors. Male or nonparous female donors are preferred over parous female donors.[60,65]
Rarely can a donor/recipient pair fit perfectly into this algorithm, and determining which of these characteristics should be chosen over others has been controversial. A CIBMTR study involving 6,349 patients who underwent transplantation for hematological malignancies from 1988 to 2006, with a confirmation cohort of 4,690 patients who underwent transplantation between 2007 and 2011, tested the effect of donor characteristics while adjusting for disease risk and other key transplant characteristics. The earlier data set showed that in addition to HLA mismatching, older donor age and major or minor ABO blood-type mismatching increased overall mortality; parous female graft recipients experienced lower rates of relapse; recipients of younger donor grafts had lower rates of acute GVHD, and recipients of parous female grafts had higher rates of chronic GVHD. Recipient CMV status was more important than donor CMV status (recipients who are CMV-positive are at higher risk of mortality independent of the donor CMV status), although a CMV-negative donor to CMV-negative recipient combination improves survival. The more-recent confirmation cohort was tested by a multivariate analysis for independent predictors of survival. Older donor age was confirmed to be independently associated with worse OS; every 10 years of donor age increased the risk of mortality by 5.5%. HLA matching continued to have the most important effect on survival; ABO mismatching was not confirmed to have a continuing effect.[60] Thus, after HLA matching, donor age is likely the most important second factor to optimize, unless the recipient is CMV-negative, at which point finding a CMV-negative donor would take priority. A number of studies have attempted to identify characteristics of the best donors for haploidentical procedures. As with conventional bone marrow transplantation, use of younger donors appears to be beneficial, but data regarding donor gender are inconclusive. Studies involving intense T-cell depletion have noted better outcomes using maternal donors,[66] but studies using posttransplant cyclophosphamide or intense immune suppression seem to favor male donors.[67,68] Further study is needed to clarify this important issue. Immunotherapeutic Effects of Allogeneic HCT Graft-versus-leukemia (GVL) effect Early studies in HCT focused on delivery of intense myeloablative preparative regimens followed by rescue of the hematopoietic system with either an autologous or allogeneic bone marrow. Investigators quickly showed that allogeneic approaches led to a decreased risk of relapse caused by an immunotherapeutic reaction of the new bone marrow graft against tumor antigens. This phenomenon came to be termed the GVL or graft-versus-tumor (GVT) effect, and has been shown to be associated with mismatches to both major and minor HLA antigens. The GVL effect is challenging to use therapeutically because of a strong association between GVL and clinical graft-versus-host disease (GVHD). For standard approaches to HCT, the highest survival rates have been associated with mild or moderate GVHD (grades I to II in AML and grades I to III in ALL), compared with patients who have no GVHD and experience more relapse or patients with severe GVHD who experience more transplant-related mortality.[69,70]; [71][Level of evidence: 3iDi] Understanding when GVL occurs and how to use GVL optimally is challenging. One method of study is comparing rates of relapse and survival between patients undergoing myeloablative HCT with either autologous or allogeneic donors for a given disease. - Leukemia and MDS: A clear advantage has been noted when allogeneic approaches are used for ALL, AML, chronic myelogenous leukemia (CML), and MDS. For ALL and AML specifically, autologous HCT approaches for most high-risk patient groups have shown results similar to those obtained with chemotherapy, while allogeneic approaches produced superior results.[72,73]
- Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL): Patients with HL or NHL generally fare better with autologous approaches, although there may be a role for allogeneic approaches in relapsed lymphoblastic lymphoma, lymphoma that is poorly responsive to chemotherapy, or lymphoma that has relapsed after autologous HCT.[74]
Further insights into the therapeutic benefit of GVL/GVT for given diseases have come from the use of reduced-intensity preparative regimens (refer to the Principles of Allogeneic HCT Preparative Regimens section of this summary for more information). This approach to transplantation relies on GVL because the intensity of the preparative regimen is not sufficient for cure in most cases. Although studies have shown benefit for patients pursuing this approach when they are ineligible for standard transplantation,[75] because pediatric cancer patients can generally undergo myeloablative approaches safely, this approach has not been used for most children with cancer who require HCT. Using donor lymphocyte infusions (DLI) or early withdrawal of immune suppression to enhance GVL One can deliver GVL therapeutically through infusion of cells after transplant that either specifically or nonspecifically target tumor. The most common approach is the use of DLI. This approach relies on the persistence of donor T-cell engraftment after transplant to prevent rejection of donor lymphocytes infused to induce the GVL. Therapeutic DLI results in potent responses in patients with CML who relapse after transplant (60%-80% enter into long-term remission),[76] but responses in other diseases (AML and ALL) have been less potent, with only 20% to 30% long-term survival.[77] DLI works poorly in patients with acute leukemia who relapse early and who have high levels of active disease. Late relapse (>6 months after transplant) and treatment of patients into complete remission with chemotherapy before DLI have been associated with improved outcomes.[78] Infusions of DLI modified to enhance GVL or other donor cells (natural killer [NK] cells, etc.) have also been studied, but have yet to be generally adopted. Another method of delivering GVL therapeutically is the rapid withdrawal of immune suppression after HCT. Some studies have scheduled more rapid immune suppression tapers based on donor type (related donors are tapered more quickly than are unrelated donors because of less GVHD risk), and others have used sensitive measures of either low levels of persistent recipient cells (recipient chimerism) or MRD to assess risk of relapse and trigger rapid taper of immune suppression. A combination of early withdrawal of immune suppression after HCT with addition of DLI to prevent relapse in patients at high risk of relapse due to persistent/progressive recipient chimerism has been tested in patients transplanted for both ALL and AML.[79][Level of evidence: 2A] For patients with ALL, one study found increasing recipient chimerism in 46 of 101 patients. Thirty-one of those patients had withdrawal of immune suppression, and a portion went on to receive DLI if GVHD did not occur. This group had a 37% survival rate, compared with 0% in the 15 patients who did not undergo this approach (P < .001).[80] For patients with AML after HCT, about 20% experienced mixed chimerism after HCT and were identified as high risk. Of these, 54% survived if they underwent withdrawal of immune suppression with or without DLI; there were no survivors among those who did not receive this therapy.[81] Other approaches under evaluation The role of killer immunoglobulin-like receptor mismatching in HCT Donor-derived NK cells in the post-HCT setting have been shown to promote the following:[82,83,84] - Engraftment.
- Decreased GVHD.
- Fewer relapses of hematological malignancies.
- Improved survival.
NK cell function is modulated by interactions with a number of receptor families, including activating and inhibiting killer immunoglobulin-like receptors. The killer immunoglobulin-like receptor effect in the allogeneic HCT setting hinges on expression of specific inhibitory killer immunoglobulin-like receptors on donor-derived NK cells and either the presence or the absence of their matching HLA class I molecules (killer immunoglobulin-like receptor ligands) on recipient leukemic and normal cells. Normally, the presence of specific killer immunoglobulin-like receptor ligands interacting with paired inhibitory killer immunoglobulin-like receptor molecules prevents NK cell attack on healthy cells. In the allogeneic transplant setting, recipient leukemia cells genetically differ from donor NK cells, and they may not have the appropriate inhibitory killer immunoglobulin-like receptor ligand. Mismatch of ligand and receptor allows NK cell-based killing of recipient leukemia cells to proceed for certain donor-recipient genetic combinations. The original observation of decreased relapse with certain killer immunoglobulin-like receptor-ligand combinations was made in the setting of T-cell-depleted haploidentical transplantation and was strongest after HCT for AML.[83,85] Along with decreasing relapse, these studies have suggested a decrease in GVHD with appropriate killer immunoglobulin-like receptor-ligand combinations. Many subsequent studies did not detect survival effects for killer immunoglobulin-like receptor-incompatible HCT using standard transplantation methods,[86,87] which has led to the conclusion that T-cell depletion may be necessary to remove other forms of inhibitory cellular interactions. Decreased relapse and better survival have been noted with donor/recipient killer immunoglobulin-like receptor-ligand incompatibility after cord blood HCT, a relatively T-cell-depleted procedure.[88,89] In contrast to this notion, one study demonstrated that some killer immunoglobulin-like receptor mismatching combinations (activating receptor KIR2DS1 with the HLA C1 ligand) can lead to decreased relapse after AML HCT without T-cell depletion.[90] The role of killer immunoglobulin-like receptor incompatibility in sibling donor HCT and in diseases other than AML is controversial, but in pediatrics, at least two groups have found better outcomes with specific types of killer immunoglobulin-like receptor mismatching in ALL.[51,91,92] A current challenge associated with studies of killer immunoglobulin-like receptor is that several different approaches have been used to determine what is killer immunoglobulin-like receptor compatible and incompatible.[85,93] Standardization of classification and prospective studies should help clarify the utility and importance of this approach. Because a limited number of centers perform haploidentical HCT and the results of the data in cord blood HCT are preliminary, most transplant programs do not use killer immunoglobulin-like receptor mismatching as part of their strategy for choosing a donor. Full HLA matching is considered most important for outcome, with considerations of killer immunoglobulin-like receptor incompatibility remaining secondary. NK cell transplantation With low risk of GVHD and demonstrated efficacy in decreasing relapse in posthaploidentical HCT settings, NK cell infusions have been studied as a method of treating high-risk patients and consolidating patients in remission. The University of Minnesota group initially failed to demonstrate efficacy with autologous NK cells, but found that intense immunoablative therapy followed by purified haploidentical NK cells and interleukin-2 (IL-2) maintenance led to remission in 5 of 19 high-risk AML patients.[94] Researchers at St. Jude Children's Research Hospital treated ten intermediate-risk AML patients who had completed chemotherapy and were in remission with lower-dose immunosuppression followed by haploidentical NK cell infusions and IL-2 for consolidation.[95] Expansion of NK cells was noted in all nine of the killer immunoglobulin-like receptor-incompatible donor/recipient pairs. All ten children remained in remission at 2 years. A follow-up phase II study is under way, as are many investigations into NK cell therapy for a number of cancer types. Chimeric antigen receptor (CAR) T-cell therapy In order for T cells to attack cellular targets (viruses or cancer cells), they must to bind to class I major histocompatibility complex (MHC) molecules on the surface of the target cells and avoid suppressor signals sent by regulatory T cells and other surface molecule interactions. Gene transfer technologies can modify T cells to express MHC-independent antibody-binding domains (CAR molecules) aimed at specific target proteins on the surface of tumors. To minimize the chance of suppressor mechanisms affecting CAR T-cell function, lymphodepleting chemotherapy is generally given before CAR T-cell infusions. CAR T-cell-mediated responses can be further enhanced by the addition of intracellular costimulatory domains (e.g.., CD28, 4-1BB), which cause significant CAR T-cell expansion and may increase the lifespan of these cells in the recipient.[96] Use of this technology has targeted a variety of tumors/surface molecules but the best-studied experience has been CAR T cells aimed at CD19, a surface receptor on B cells. Several groups have reported significant rates of remission (70%-90%) in children and adults with refractory B-cell ALL,[97,98,99] and one group has reported persistence of CAR T cells and remission beyond 6 months in most patients studied.[100] Responses have been associated with a significant increase in inflammatory cytokines (termed cytokine release syndrome) that has given a sepsis-like picture that can be successfully treated with anti-IL-6 therapies (tocilizumab).[101] Early loss of the CAR T cells is associated with relapse, and the best use of this therapy (bridge to transplant vs. definitive therapy) is under study. Principles of Allogeneic HCT Preparative Regimens In the days before infusion of the stem cell product (bone marrow, peripheral blood stem cells, or cord blood), HCT recipients receive chemotherapy/immunotherapy, sometimes combined with radiation therapy. This is called a preparative regimen, and the original intent of this treatment was to: - Create bone marrow space in the recipient for the donor cells to engraft.
- Suppress the immune system or eliminate the recipient T cells to minimize risks of rejection.
- Intensely treat cancer (if present) with mega-dose therapy of active agents, with the intent to overcome therapy resistance.
With the recognition that donor T cells can facilitate engraftment and kill tumors through GVL effects (obviating the need to create bone marrow space and intensely treat cancer), reduced-intensity or minimal-intensity HCT approaches focusing on immune suppression rather than myeloablation have been developed. The resultant lower toxicity associated with these regimens has led to lower rates of transplant-related mortality and an expanded eligibility for allogeneic HCT to older individuals and younger patients with pre-HCT comorbidities that put them at risk of severe toxicity after standard HCT approaches.[102] The preparative regimens available now vary tremendously in the amount of immunosuppression and myelosuppression that they cause, with the lowest-intensity regimens relying heavily on a strong graft-versus-tumor effect.
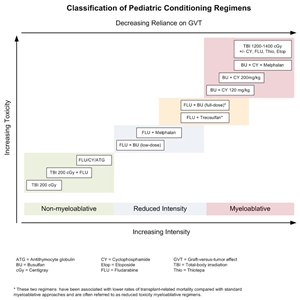
Figure 3. Selected preparative regimens frequently used in pediatric HCT categorized by current definitions as nonmyeloablative, reduced-intensity, or myeloablative. Although FLU plus treosulfan and FLU plus busulfan (full-dose) are considered myeloablative approaches, some refer to them as reduced-toxicity regimens.
Although these regimens represent a spectrum of varying degrees of myelosuppression and immune suppression, they have been grouped clinically into the following three major categories (refer to Figure 4):[103] - Myeloablative: Intense approaches that cause irreversible pancytopenia that requires stem cell rescue for restoration of hematopoiesis.
- Nonmyeloablative: Regimens that cause minimal cytopenias and do not require stem cell support.
- Reduced-intensity conditioning: Regimens that are of intermediate intensity and do not meet the definitions of nonmyeloablative or myeloablative regimens.
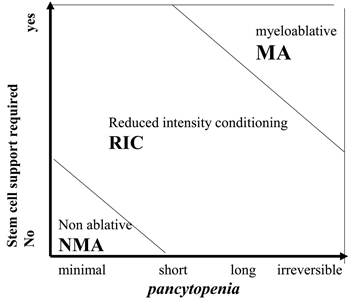
Figure 4. Classification of conditioning regimens in 3 categories, based on duration of pancytopenia and requirement for stem cell support. Myeloablative regimens (MA) produce irreversible pancytopenia and require stem cell support. Nonmyeloablative regimens (NMA) produce minimal cytopenia and would not require stem cell support. Reduced-intensity regimens (RIC) are regimens which cannot be classified as MA nor NMA. Reprinted from Biology of Blood and Marrow Transplantation, Volume 15 (Issue 12), Andrea Bacigalupo, Karen Ballen, Doug Rizzo, Sergio Giralt, Hillard Lazarus, Vincent Ho, Jane Apperley, Shimon Slavin, Marcelo Pasquini, Brenda M. Sandmaier, John Barrett, Didier Blaise, Robert Lowski, Mary Horowitz, Defining the Intensity of Conditioning Regimens: Working Definitions, Pages 1628-1633, Copyright 2009, with permission from Elsevier.
The use of reduced-intensity conditioning and nonmyeloablative regimens is well established in older adults who cannot tolerate more-intense myeloablative approaches,[104,105,106] but these approaches have been studied in only a handful of younger patients with malignancies.[107,108,109,110,111] A large Pediatric Blood and Marrow Transplant Consortium study identified patients at high risk of transplant-related mortality with myeloablative regimens (e.g., history of previous myeloablative transplant, severe organ system dysfunction, or active invasive fungal infection) and successfully treated them with a reduced-intensity regimen.[75] Transplant-related mortality was low in this high-risk group, and long-term survival occurred in most patients with minimal or no detectable disease present at the time of transplantation. Because the risks of relapse are higher with these approaches, their use in pediatric cancer is currently limited to patients ineligible for myeloablative regimens. Establishing donor chimerism Intense myeloablative approaches almost invariably result in rapid establishment of hematopoiesis derived completely from donor cells upon count recovery within weeks of the transplant. The introduction of reduced-intensity conditioning and nonmyeloablative approaches into HCT practice has resulted in a slower pace of transition to donor hematopoiesis (gradually increasing from partial to full donor hematopoiesis over months) that is sometimes only partial. DNA-based techniques have been established to differentiate donor and recipient hematopoiesis, applying the word chimerism (from the Greek chimera, a mythical animal with parts taken from various animals) to describe whether all or part of hematopoiesis after HCT is from the donor or recipient. There are several implications to the pace and extent of donor chimerism eventually achieved by an HCT recipient. For patients receiving reduced-intensity conditioning or nonmyeloablative regimens, rapid progression to full donor chimerism is associated with less relapse but more GVHD.[112] The delayed pace of obtaining full donor chimerism after these regimens has led to late-onset acute GVHD, occurring as long as 6 months to 7 months after HCT (generally within 100 days after myeloablative approaches).[113] A portion of patients achieve stable mixed chimerism of both donor and recipient. Mixed chimerism is associated with more relapse after HCT for malignancies and less GVHD; however, this condition is often advantageous for nonmalignant HCT, where usually only a percentage of normal hematopoiesis is needed to correct the underlying disorder and GVHD is not beneficial.[114] Finally, serially measured decreasing donor chimerism, especially T-cell-specific chimerism, has been associated with increased risk of rejection.[115] Because of the implications of persistent recipient chimerism, most transplant programs test for chimerism shortly after engraftment and continue testing regularly until stable full donor hematopoiesis has been achieved. Investigators have defined two approaches to treat the increased risks of relapse and rejection associated with increasing recipient chimerism: rapid withdrawal of immune suppression and DLI. (Refer to the Using donor lymphocyte infusions (DLI) or early withdrawal of immune suppression to enhance GVL section of this summary for more information.) These approaches are frequently used to address this issue, and have been shown in some cases to decrease relapse risk and stop rejection.[80,116,117] Timing of tapers of immune suppression and doses and approaches to the administration of DLI to increase or stabilize donor chimerism vary tremendously among transplant regimens and institutions. References:
-
Hahn T, McCarthy PL Jr, Hassebroek A, et al.: Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol 31 (19): 2437-49, 2013.
-
Horan JT, Logan BR, Agovi-Johnson MA, et al.: Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol 29 (7): 805-13, 2011.
-
Wood WA, Lee SJ, Brazauskas R, et al.: Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biol Blood Marrow Transplant 20 (6): 829-36, 2014.
-
MacMillan ML, Davies SM, Nelson GO, et al.: Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant 14 (9 Suppl): 16-22, 2008.
-
Harvey J, Green A, Cornish J, et al.: Improved survival in matched unrelated donor transplant for childhood ALL since the introduction of high-resolution matching at HLA class I and II. Bone Marrow Transplant 47 (10): 1294-300, 2012.
-
Majhail NS, Chitphakdithai P, Logan B, et al.: Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant 21 (1): 142-50, 2015.
-
Barker JN, Byam CE, Kernan NA, et al.: Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant 16 (11): 1541-8, 2010.
-
Gragert L, Eapen M, Williams E, et al.: HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 371 (4): 339-48, 2014.
-
Woolfrey A, Klein JP, Haagenson M, et al.: HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 17 (6): 885-92, 2011.
-
Howard CA, Fernandez-Vina MA, Appelbaum FR, et al.: Recommendations for donor human leukocyte antigen assessment and matching for allogeneic stem cell transplantation: consensus opinion of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). Biol Blood Marrow Transplant 21 (1): 4-7, 2015.
-
Shaw PJ, Kan F, Woo Ahn K, et al.: Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood 116 (19): 4007-15, 2010.
-
Flomenberg N, Baxter-Lowe LA, Confer D, et al.: Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 104 (7): 1923-30, 2004.
-
Petersdorf EW, Kollman C, Hurley CK, et al.: Effect of HLA class II gene disparity on clinical outcome in unrelated donor hematopoietic cell transplantation for chronic myeloid leukemia: the US National Marrow Donor Program Experience. Blood 98 (10): 2922-9, 2001.
-
Fernandez-Viña MA, Wang T, Lee SJ, et al.: Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood 123 (8): 1270-8, 2014.
-
Fernández-Viña MA, Klein JP, Haagenson M, et al.: Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood 121 (22): 4603-10, 2013.
-
Fleischhauer K, Shaw BE, Gooley T, et al.: Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol 13 (4): 366-74, 2012.
-
Crocchiolo R, Zino E, Vago L, et al.: Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood 114 (7): 1437-44, 2009.
-
Pidala J, Lee SJ, Ahn KW, et al.: Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood 124 (16): 2596-606, 2014.
-
Spellman S, Bray R, Rosen-Bronson S, et al.: The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood 115 (13): 2704-8, 2010.
-
Ciurea SO, Thall PF, Wang X, et al.: Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood 118 (22): 5957-64, 2011.
-
Hurley CK, Woolfrey A, Wang T, et al.: The impact of HLA unidirectional mismatches on the outcome of myeloablative hematopoietic stem cell transplantation with unrelated donors. Blood 121 (23): 4800-6, 2013.
-
Eapen M, Rubinstein P, Zhang MJ, et al.: Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369 (9577): 1947-54, 2007.
-
Eapen M, Klein JP, Ruggeri A, et al.: Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood 123 (1): 133-40, 2014.
-
Takanashi M, Atsuta Y, Fujiwara K, et al.: The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood 116 (15): 2839-46, 2010.
-
Cutler C, Kim HT, Sun L, et al.: Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood 118 (25): 6691-7, 2011.
-
Rocha V, Spellman S, Zhang MJ, et al.: Effect of HLA-matching recipients to donor noninherited maternal antigens on outcomes after mismatched umbilical cord blood transplantation for hematologic malignancy. Biol Blood Marrow Transplant 18 (12): 1890-6, 2012.
-
van Rood JJ, Stevens CE, Smits J, et al.: Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci U S A 106 (47): 19952-7, 2009.
-
Kanda J, Atsuta Y, Wake A, et al.: Impact of the direction of HLA mismatch on transplantation outcomes in single unrelated cord blood transplantation. Biol Blood Marrow Transplant 19 (2): 247-54, 2013.
-
Stevens CE, Carrier C, Carpenter C, et al.: HLA mismatch direction in cord blood transplantation: impact on outcome and implications for cord blood unit selection. Blood 118 (14): 3969-78, 2011.
-
Cunha R, Loiseau P, Ruggeri A, et al.: Impact of HLA mismatch direction on outcomes after umbilical cord blood transplantation for hematological malignant disorders: a retrospective Eurocord-EBMT analysis. Bone Marrow Transplant 49 (1): 24-9, 2014.
-
Barker JN, Rocha V, Scaradavou A: Optimizing unrelated donor cord blood transplantation. Biol Blood Marrow Transplant 15 (1 Suppl): 154-61, 2009.
-
Wagner JE, Barker JN, DeFor TE, et al.: Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100 (5): 1611-8, 2002.
-
Rubinstein P, Carrier C, Scaradavou A, et al.: Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 339 (22): 1565-77, 1998.
-
Barker JN, Weisdorf DJ, DeFor TE, et al.: Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105 (3): 1343-7, 2005.
-
Michel G, Galambrun C, Sirvent A, et al.: Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood 127 (26): 3450-7, 2016.
-
Wagner JE Jr, Eapen M, Carter S, et al.: One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 371 (18): 1685-94, 2014.
-
Bensinger WI, Martin PJ, Storer B, et al.: Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 344 (3): 175-81, 2001.
-
Rocha V, Cornish J, Sievers EL, et al.: Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 97 (10): 2962-71, 2001.
-
Bertaina A, Merli P, Rutella S, et al.: HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 124 (5): 822-6, 2014.
-
Eapen M, Horowitz MM, Klein JP, et al.: Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol 22 (24): 4872-80, 2004.
-
Shinzato A, Tabuchi K, Atsuta Y, et al.: PBSCT is associated with poorer survival and increased chronic GvHD than BMT in Japanese paediatric patients with acute leukaemia and an HLA-matched sibling donor. Pediatr Blood Cancer 60 (9): 1513-9, 2013.
-
Anasetti C, Logan BR, Lee SJ, et al.: Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367 (16): 1487-96, 2012.
-
Milano F, Gooley T, Wood B, et al.: Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 375 (10): 944-53, 2016.
-
Ruggeri A, Michel G, Dalle JH, et al.: Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia 26 (12): 2455-61, 2012.
-
Bachanova V, Burke MJ, Yohe S, et al.: Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant 18 (6): 963-8, 2012.
-
Sutton R, Shaw PJ, Venn NC, et al.: Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol 168 (3): 395-404, 2015.
-
Sanchez-Garcia J, Serrano J, Serrano-Lopez J, et al.: Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant 48 (3): 396-402, 2013.
-
Beatty PG, Clift RA, Mickelson EM, et al.: Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med 313 (13): 765-71, 1985.
-
Aversa F, Tabilio A, Velardi A, et al.: Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 339 (17): 1186-93, 1998.
-
Barrett J, Gluckman E, Handgretinger R, et al.: Point-counterpoint: haploidentical family donors versus cord blood transplantation. Biol Blood Marrow Transplant 17 (1 Suppl): S89-93, 2011.
-
Leung W, Campana D, Yang J, et al.: High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 118 (2): 223-30, 2011.
-
González-Vicent M, Molina B, Andión M, et al.: Allogeneic hematopoietic transplantation using haploidentical donor vs. unrelated cord blood donor in pediatric patients: a single-center retrospective study. Eur J Haematol 87 (1): 46-53, 2011.
-
Handgretinger R, Chen X, Pfeiffer M, et al.: Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci 1106: 279-89, 2007.
-
Leen AM, Christin A, Myers GD, et al.: Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 114 (19): 4283-92, 2009.
-
Huang XJ, Liu DH, Liu KY, et al.: Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38 (4): 291-7, 2006.
-
Luznik L, Fuchs EJ: High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 47 (1-3): 65-77, 2010.
-
Pulsipher MA, Chitphakdithai P, Logan BR, et al.: Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood 114 (13): 2606-16, 2009.
-
Aversa F, Terenzi A, Tabilio A, et al.: Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 23 (15): 3447-54, 2005.
-
Kollman C, Howe CW, Anasetti C, et al.: Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98 (7): 2043-51, 2001.
-
Kollman C, Spellman SR, Zhang MJ, et al.: The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 127 (2): 260-7, 2016.
-
Seebach JD, Stussi G, Passweg JR, et al.: ABO blood group barrier in allogeneic bone marrow transplantation revisited. Biol Blood Marrow Transplant 11 (12): 1006-13, 2005.
-
Logan AC, Wang Z, Alimoghaddam K, et al.: ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 21 (4): 746-54, 2015.
-
Stussi G, Muntwyler J, Passweg JR, et al.: Consequences of ABO incompatibility in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 30 (2): 87-93, 2002.
-
Boeckh M, Nichols WG: The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103 (6): 2003-8, 2004.
-
Loren AW, Bunin GR, Boudreau C, et al.: Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 12 (7): 758-69, 2006.
-
Stern M, Ruggeri L, Mancusi A, et al.: Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 112 (7): 2990-5, 2008.
-
Ciurea SO, Champlin RE: Donor selection in T cell-replete haploidentical hematopoietic stem cell transplantation: knowns, unknowns, and controversies. Biol Blood Marrow Transplant 19 (2): 180-4, 2013.
-
Wang Y, Chang YJ, Xu LP, et al.: Who is the best donor for a related HLA haplotype-mismatched transplant? Blood 124 (6): 843-50, 2014.
-
Pulsipher MA, Langholz B, Wall DA, et al.: The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood 123 (13): 2017-25, 2014.
-
Neudorf S, Sanders J, Kobrinsky N, et al.: Allogeneic bone marrow transplantation for children with acute myelocytic leukemia in first remission demonstrates a role for graft versus leukemia in the maintenance of disease-free survival. Blood 103 (10): 3655-61, 2004.
-
Boyiadzis M, Arora M, Klein JP, et al.: Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin Cancer Res 21 (9): 2020-8, 2015.
-
Woods WG, Neudorf S, Gold S, et al.: A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood 97 (1): 56-62, 2001.
-
Ribera JM, Ortega JJ, Oriol A, et al.: Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol 25 (1): 16-24, 2007.
-
Gross TG, Hale GA, He W, et al.: Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant 16 (2): 223-30, 2010.
-
Pulsipher MA, Boucher KM, Wall D, et al.: Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood 114 (7): 1429-36, 2009.
-
Porter DL, Collins RH Jr, Shpilberg O, et al.: Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant 5 (4): 253-61, 1999.
-
Levine JE, Barrett AJ, Zhang MJ, et al.: Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant 42 (3): 201-5, 2008.
-
Warlick ED, DeFor T, Blazar BR, et al.: Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 18 (3): 480-6, 2012.
-
Horn B, Petrovic A, Wahlstrom J, et al.: Chimerism-based pre-emptive immunotherapy with fast withdrawal of immunosuppression and donor lymphocyte infusions after allogeneic stem cell transplantation for pediatric hematologic malignancies. Biol Blood Marrow Transplant 21 (4): 729-37, 2015.
-
Bader P, Kreyenberg H, Hoelle W, et al.: Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 22 (9): 1696-705, 2004.
-
Rettinger E, Willasch AM, Kreyenberg H, et al.: Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 118 (20): 5681-8, 2011.
-
Ruggeri L, Capanni M, Urbani E, et al.: Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295 (5562): 2097-100, 2002.
-
Giebel S, Locatelli F, Lamparelli T, et al.: Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 102 (3): 814-9, 2003.
-
Bari R, Rujkijyanont P, Sullivan E, et al.: Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 31 (30): 3782-90, 2013.
-
Ruggeri L, Mancusi A, Capanni M, et al.: Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 110 (1): 433-40, 2007.
-
Davies SM, Ruggieri L, DeFor T, et al.: Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood 100 (10): 3825-7, 2002.
-
Farag SS, Bacigalupo A, Eapen M, et al.: The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 12 (8): 876-84, 2006.
-
Cooley S, Trachtenberg E, Bergemann TL, et al.: Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 113 (3): 726-32, 2009.
-
Willemze R, Rodrigues CA, Labopin M, et al.: KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia 23 (3): 492-500, 2009.
-
Venstrom JM, Pittari G, Gooley TA, et al.: HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 367 (9): 805-16, 2012.
-
Leung W: Use of NK cell activity in cure by transplant. Br J Haematol 155 (1): 14-29, 2011.
-
Oevermann L, Michaelis SU, Mezger M, et al.: KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood 124 (17): 2744-7, 2014.
-
Leung W, Iyengar R, Triplett B, et al.: Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol 174 (10): 6540-5, 2005.
-
Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al.: Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105 (8): 3051-7, 2005.
-
Rubnitz JE, Inaba H, Ribeiro RC, et al.: NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 28 (6): 955-9, 2010.
-
Kalos M, Levine BL, Porter DL, et al.: T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3 (95): 95ra73, 2011.
-
Grupp SA, Kalos M, Barrett D, et al.: Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368 (16): 1509-18, 2013.
-
Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al.: T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385 (9967): 517-28, 2015.
-
Davila ML, Riviere I, Wang X, et al.: Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6 (224): 224ra25, 2014.
-
Maude SL, Frey N, Shaw PA, et al.: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371 (16): 1507-17, 2014.
-
Lee DW, Gardner R, Porter DL, et al.: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124 (2): 188-95, 2014.
-
Deeg HJ, Sandmaier BM: Who is fit for allogeneic transplantation? Blood 116 (23): 4762-70, 2010.
-
Bacigalupo A, Ballen K, Rizzo D, et al.: Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15 (12): 1628-33, 2009.
-
Giralt S, Estey E, Albitar M, et al.: Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 89 (12): 4531-6, 1997.
-
Slavin S, Nagler A, Naparstek E, et al.: Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91 (3): 756-63, 1998.
-
Storb R, Yu C, Sandmaier BM, et al.: Mixed hematopoietic chimerism after marrow allografts. Transplantation in the ambulatory care setting. Ann N Y Acad Sci 872: 372-5; discussion 375-6, 1999.
-
Bradley MB, Satwani P, Baldinger L, et al.: Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant 40 (7): 621-31, 2007.
-
Del Toro G, Satwani P, Harrison L, et al.: A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant 33 (6): 613-22, 2004.
-
Gómez-Almaguer D, Ruiz-Argüelles GJ, Tarín-Arzaga Ldel C, et al.: Reduced-intensity stem cell transplantation in children and adolescents: the Mexican experience. Biol Blood Marrow Transplant 9 (3): 157-61, 2003.
-
Pulsipher MA, Woolfrey A: Nonmyeloablative transplantation in children. Current status and future prospects. Hematol Oncol Clin North Am 15 (5): 809-34, vii-viii, 2001.
-
Roman E, Cooney E, Harrison L, et al.: Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin Cancer Res 11 (19 Pt 2): 7164s-7170s, 2005.
-
Baron F, Baker JE, Storb R, et al.: Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 104 (8): 2254-62, 2004.
-
Vigorito AC, Campregher PV, Storer BE, et al.: Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood 114 (3): 702-8, 2009.
-
Marsh RA, Vaughn G, Kim MO, et al.: Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood 116 (26): 5824-31, 2010.
-
McSweeney PA, Niederwieser D, Shizuru JA, et al.: Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 97 (11): 3390-400, 2001.
-
Horn B, Soni S, Khan S, et al.: Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant 43 (6): 469-76, 2009.
-
Haines HL, Bleesing JJ, Davies SM, et al.: Outcomes of donor lymphocyte infusion for treatment of mixed donor chimerism after a reduced-intensity preparative regimen for pediatric patients with nonmalignant diseases. Biol Blood Marrow Transplant 21 (2): 288-92, 2015.
Complications After HCTPre-HCT Comorbidities That Affect the Risk of Transplant-Related Mortality: Predictive Power of the HCT-Specific Comorbidity Index Because of the intensity of therapy associated with the transplant process, the pretransplant clinical status of recipients (e.g., age, presence of infections or organ dysfunction, and functional status) is associated with risk of transplant-related mortality. The best tool to assess the impact of pretransplant comorbidities on outcomes after transplant was developed by adapting an existing comorbidity scale, the Charlson Comorbidity Index (CCI). Investigators at the Fred Hutchinson Cancer Research Center systematically defined which of the CCI elements were correlated with transplant-related mortality in adult and pediatric patients. They also determined several additional comorbidities that have predictive power specific to transplant patients. Successful validation defined what is now termed the hematopoietic cell transplantation-specific comorbidity index (HCT-CI).[1,2] Transplant-related mortality increases with cardiac, hepatic, pulmonary, gastrointestinal, infectious, and autoimmune comorbidities, or a history of previous solid tumors (refer to Table 4). Table 4. Definitions of Comorbidities Included in the Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI)a| HCT-CI Score |
|---|
| AST/ALT = aspartate aminotransferase/alanine aminotransferase; DLCO = diffusion capacity of carbon monoxide; FEV1 = forced expiratory volume in one second; ULN = upper limit of normal. | | a Adapted from Sorror et al.[1] | | b One-or-more-vessel coronary artery stenosis requiring medical treatment, stent, or bypass graft. | | 1 | 2 | 3 | | Arrhythmia: Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | Moderate pulmonary: DLCO and/or FEV1 66%-80% or dyspnea on slight activity | Heart valve disease: Excluding mitral valve prolapse | | Cardiac: Coronary artery disease,b congestive heart failure, myocardial infarction, or ejection fraction ≤50% | Moderate/severe renal: Serum creatinine >2 mg/dL, on dialysis, or prior renal transplantation | Moderate/severe hepatic: Liver cirrhosis, bilirubin >1.5 × ULN, or AST/ALT >2.5 × ULN | | Cerebrovascular disease: Transient ischemic attack or cerebrovascular accident | Peptic ulcer: Requiring treatment | Prior solid tumor: Treated at any time in the patient's history, excluding nonmelanoma skin cancer | | Diabetes: Requiring treatment with insulin or oral hypoglycemic agents but not diet alone | Rheumatologic: Systemic lupus erythematosus, rheumatoid arthritis, polymyositis, mixed connective tissue disease, or polymyalgia rheumatica | Severe pulmonary: DLCO and/or FEV1 <65% or dyspnea at rest or requiring oxygen | | Hepatic, mild: Chronic hepatitis, bilirubin > ULN or AST/ALT > ULN to 2.5 × ULN | | | | Infection: Requiring continuation of antimicrobial treatment after day 0 | | | | Inflammatory bowel disease: Crohn disease or ulcerative colitis | | | | Obesity: Body mass index >35 kg/m2 | | | | Psychiatric disturbance: Depression or anxiety requiring psychiatric consult or treatment | | | The predictive power of this index for both transplant-related mortality and overall survival (OS) is strong, with a hazard ratio of 3.54 (95% confidence interval [CI], 2.0-6.3) for nonrelapse mortality and 2.69 (95% CI, 1.8-4.1) for survival for patients with a score of 3 or higher, compared with those who have a score of 0. Although the original studies were performed with patients receiving intense myeloablative approaches, the HCT-CI has also been shown to be predictive of outcome for patients receiving reduced-intensity and nonmyeloablative regimens.[3] It has also been combined with disease status [4] and Karnofsky score,[5] leading to even better prediction of survival outcomes. In addition, high HCT-CI scores (>3) have been associated with a higher risk of grades 3 to 4 acute graft-versus-host disease (GVHD).[6] Most patients assessed in the HCT-CI studies have been adults, and the comorbidities listed are skewed toward adult diseases. The relevance of this scale for pediatric and young adult recipients of HCT has been explored in the following studies: - A retrospective cohort study was conducted at four large centers of pediatric patients (median age of 6 years) with a wide variety of both malignant and nonmalignant disorders.[7] The HCT-CI was predictive of both nonrelapse mortality and survival, with 1-year nonrelapse mortality of 10%, 14%, and 28% and 1-year OS of 88%, 67%, and 62% for patients with scores of 0, 1 to 2, and 3 or higher, respectively.
- A second study included young adults (aged 16-39 years) and demonstrated similar increases in mortality with higher HCT-CI scores (nonrelapse mortality of 24% and 38% and OS of 46% and 28% for patients with scores of 0-2 and 3+, respectively).[8]
- As part of a prospective validation of the HCT-CI through the Center for International Blood and Marrow Transplant Research, 23,876 patients-including 1,755 children-transplanted between 2007 and 2009 were scored and outcomes were tracked. Although adults treated with myeloablative regimens had increased mortality with scores of 1 or 2, pediatric patients did not have increased mortality until a score of 3 or more was noted.[9]
Most of the reported comorbidities in these studies were with respiratory or hepatic conditions and infection.[7,8] In the adolescent and young adult study, patients with pre-HCT pulmonary dysfunction were at particularly high risk of comorbidity, with a 2-year OS of 29%, compared with 61% in those with normal lung function before HCT.[8] Selected HCT-Related Acute Complications Infectious risks and immune recovery after transplantation Figure 5 illustrates the immune defects, contributing transplant-related factors, and types and timing of infections that occur after allogeneic transplantation.[10] Defective immune reconstitution is a major barrier to successful HCT, regardless of graft source.[11,12] Serious infections have been shown to account for a significant percentage (4%-20%) of late deaths after HCT.[13] Factors that can significantly slow immune recovery include graft manipulation (removal of T cells), stem cell source (slow recovery with cord blood), and chronic graft-versus-host disease (GVHD).[14]
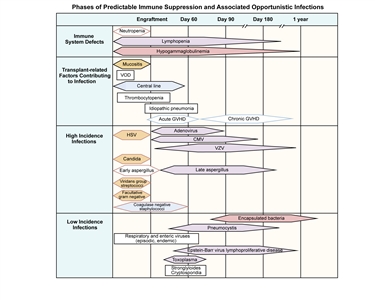
Figure 5. Phases of predictable immune suppression with their opportunistic infections among allogeneic hematopoietic stem cell transplantation recipients. Adapted from Burik and Freifeld. This figure was published in Clinical Oncology, 3rd edition, Abeloff et al., Chapter: Infection in the severely immunocompromised patient, Pages 941-956, Copyright Elsevier (2004). Bacterial infections tend to occur in the first few weeks after transplant during the neutropenic phase, when mucosal barriers are damaged from the conditioning regimen; there is significant ongoing study about the role of prophylactic antibacterial medications during the neutropenic phase.[15] Prophylaxis against fungal infections is standard during the first several months after transplantation and may be considered for patients with chronic GVHD who are at high risk of fungal infection. Antifungal prophylaxis must be tailored to the patient's underlying immune status. Pneumocystis infection can occur in all patients post-bone marrow transplant, and prophylaxis is mandatory.[15] After HCT, viral infections can be a major source of mortality, especially after T-cell-depleted or cord blood procedures. Cytomegalovirus (CMV) infection has been a major cause of mortality in the past, but effective drugs to treat CMV are available, and preventive strategies, including quantitative polymerase chain reaction (PCR) monitoring followed by preemptive therapy with ganciclovir, have been developed. Epstein-Barr virus (EBV) rarely causes lymphoproliferative disease and is generally associated with intensive, multidrug GVHD therapy or T-cell-depleted HCT. Adenovirus infection is a major issue in T-cell-depleted transplantation, and monitoring by quantitative blood PCR followed by cidofovir therapy has led to a major decrease in morbidity. Other viruses have been implicated in hemorrhagic cystitis (BK virus), encephalitis and poor count recovery (human herpes virus 6), and other clinical issues. Careful viral monitoring is essential during high-risk allogeneic procedures.[15] Late bacterial infections can occur in patients who have central lines or patients with significant chronic GVHD. These patients are susceptible to infection with encapsulated organisms, particularly pneumococcus. Despite reimmunization, these patients can sometimes develop significant infections, and continued prophylaxis is recommended until a serological response to immunizations has been documented. Occasionally, postallogeneic HCT patients can become functionally asplenic, and antibiotic prophylaxis is recommended. Patients should remain on infection prophylaxis (e.g., PCP prophylaxis) until immune recovery. Time to immune recovery varies, but ranges from 3 months to 9 months after autologous HCT and 9 months to 24 months after allogeneic HCT without GVHD. Patients with active chronic GVHD may have persistent immunosuppression for years. Many centers monitor T-cell subset recovery post-bone marrow transplant as a guide to infection risk.[15] Vaccination after transplantation Specific guidelines have been developed by international transplant and infectious disease groups for administration of vaccinations after autologous and allogeneic transplantation (refer to Table 5).[15] Autologous transplant recipients should receive immunizations beginning at 6 months after stem cell infusion and should receive live vaccines 24 months after the transplant. Patients undergoing allogeneic procedures can begin immunizations as soon as 6 months after transplant. However, many groups prefer to wait either until 12 months after the procedure for patients remaining on immune suppression or until patients are off immune suppression. Comparative studies aimed at defining ideal timing of vaccination after transplantation have not been performed, but the vaccine guidelines outlined in Table 5 result in protective titers in most patients who receive vaccinations. Table 5. Vaccination Schedule for Hematopoietic Stem Cell Transplantation (HSCT) Recipientsa| Autologous HSCT | 6 Mob | 8 Mob | 12 Mob | 24 Mob |
|---|
| Allogeneic HSCT (if not immunized prior to 12 mo post-HSCT; start regardless of GVHD status or immunosuppression) | 12 mob(sooner if off immunosuppression) | 14 mob(or 2 mo after first dose) | 18 mob(or 6 mo after first dose) | 24 mob |
|---|
| GVHD = graft-versus-host disease; IM = intramuscular; PO = orally. | | a Adapted from Tomblyn et al.,[15]Centers for Disease Control and Prevention,[16]and Kumar et al.[17] | | b Times indicated are times posttransplant (day 0). | | c Ok to use Tdap if DTap is not available. | | d Titers may be considered for pediatric patients and patients with GVHD who received immunizations while on immune suppression (minimum 6-8 weeks after last vaccination). | | e May start as soon as 4 months post-HSCT or sooner for patients with CD4 counts >200/mcL or at any time during an epidemic. If given <6 months after HSCT, may require second dose. Children younger than 9 years require second dose, separated by 1 month. | | f Consider pre- or postvaccine (at least 6-8 weeks after) titers. | | g PCV 7 at 24 months only for patients with GVHD; all other patients can get PPV 23. | | h Pediatric patients should receive two doses at least 1 month apart. | | Inactivated Vaccines | | Diphtheria, tetanus, acellular pertussis (DTap) | Xc | Xc | Xc,d | | | Haemophilus influenzae (Hib) | X | X | Xd | | | Hepatitis B (HepB) | X | X | Xd | | | Inactive polio (IPV) | X | X | Xd | | | Influenza-seasonal injection (IM) | Xe | | Pneumococcal conjugate (PCV 7, PCV 13) | Xf | X | Xd,f,g | | | Pneumococcal polysaccharide (PPV 23) | | | Xd,f,g | | | Live Attenuated Vaccines(contraindicated in patients with active GVHD or on immunosuppression) | | Measles-mumps-rubella | | | | Xd,h | | Optional Inactivated Vaccines | | Hepatitis A | | | Optional | | | Meningococcal | | | Xd(for high-risk patients) | | | Optional Live Vaccines(contraindicated in patients with active GVHD or on immunosuppression) | | Chicken pox (varicella vaccine) | | | | Optional | | Rabies | | | May be considered at 12-24 mo if exposed | | Yellow fever, tick-borne encephalitis (TBE), Japanese B encephalitis | | | | For travel in endemic areas | | Contraindicated Vaccines | | Intranasal influenza (trivalent live-attenuated influenza vaccine) -household contacts and caregivers should not receive within 2 weeks prior to contact with HSCT recipient;shingles;bacillus Calmette-Guerin (BCG);oral polio vaccine (OPV);cholera;typhoid vaccine (PO, IM);rotavirus. | Sinusoidal obstruction syndrome/veno-occlusive disease Sinusoidal obstructive syndrome/veno-occlusive disease of the liver (SOS/VOD) is defined clinically by the following: - Right upper quadrant pain with hepatomegaly.
- Fluid retention (weight gain and ascites).
- Hyperbilirubinemia.
Pathologically, the disease is the result of damage to the hepatic sinusoids, resulting in biliary obstruction. This syndrome has been estimated to occur in 15% to 40% of pediatric myeloablative transplantation patients; risk factors include the use of busulfan (especially before therapeutic pharmacokinetic monitoring), total-body irradiation, serious infection, GVHD, and pre-existing liver dysfunction due to hepatitis or iron overload.[18,19] Life-threatening SOS/VOD generally occurs soon after transplantation and is characterized by multiorgan system failure.[20] Milder, reversible forms can occur, with full recovery expected. Pediatric patients who have severe VOD without increased bilirubin have been reported;[21] therefore, it is important to be vigilant about monitoring patients who have other symptoms without increased bilirubin. Approaches to both prevention and treatment with agents such as heparin, protein C, and antithrombin III have been studied, with mixed results.[22] One small, retrospective, single-center study showed a benefit from corticosteroid therapy, but further validation is needed.[23] Another agent with demonstrated activity is defibrotide, a mixture of oligonucleotides with antithrombotic and fibrinolytic effects on microvascular endothelium. Defibrotide has been shown to decrease mortality in the treatment of severe VOD [24,25,26,27] and has also shown efficacy in decreasing VOD incidence when used prophylactically.[28][Level of evidence: 1iiA] Defibrotide is not FDA approved but is routinely used by U.S. centers through a pre-approval protocol. The British Society for Blood and Marrow Transplantation published evidence-guided recommendations on the diagnosis and management of VOD.[27] They recommend that biopsy be reserved for difficult cases and performed using the transjugular approach. They support the use of defibrotide for prevention of SOS/VOD (defibrotide prophylaxis is not currently allowed on the U.S. pre-approval protocol), but concluded there is insufficient data to support the use of prostaglandin E1, pentoxifylline, or antithrombin. For treatment of VOD, they recommend aggressive fluid balance management, early involvement of critical care and gastroenterology specialists, and the use of defibrotide and possibly methylprednisolone, but concluded there is insufficient evidence to support the use of tissue plasminogen activator or N-acetylcysteine.[27,29] Transplant-associated microangiopathy Although transplant-associated microangiopathy clinically mirrors hemolytic uremic syndrome, its causes and clinical course differ from those of other hemolytic uremic syndrome-like diseases. Studies have linked this syndrome with disruption of alternative complement pathways.[30] Transplant-associated microangiopathy has most frequently been associated with the use of the calcineurin inhibitors tacrolimus and cyclosporine, and has been noted to occur more frequently when either of these medications are used in combination with sirolimus.[31] Diagnostic criteria for this syndrome have been standardized and include the presence of schistocytes on a peripheral smear and increased lactic dehydrogenase, decreased haptoglobin, and thrombocytopenia with or without anemia.[32] Suggestive symptoms consistent with but not necessary for the diagnosis include a sudden worsening of renal function and neurologic symptoms. Treatment for transplant-associated microangiopathy includes cessation of the calcineurin inhibitor and substitution with other immune suppressants if necessary. In addition, careful management of hypertension and renal damage by dialysis, if necessary, is vital. Prognosis for normalization of kidney function when disease is caused by calcineurin inhibitors alone is generally poor; however, most transplant-associated microangiopathy associated with the combination of a calcineurin inhibitor and sirolimus has been reversed after sirolimus is stopped, and in some cases, both medications.[31] Some evidence suggests a role for complement modulation (c5, eculizumab therapy) in preserving renal function; further assessment of the role of this medication in treating this complication is ongoing.[33] Idiopathic pneumonia syndrome Idiopathic pneumonia syndrome is characterized by diffuse, noninfectious lung injury that occurs from 14 days to 90 days after the infusion of donor cells. Possible etiologies include direct toxic effects of the conditioning regimens and occult infection leading to secretion of high levels of inflammatory cytokines into the alveoli. Mortality rates of 50% to 70% have been reported;[34] however, these estimates are from the mid-1990s, and outcomes may have improved. The incidence of this complication appears to be decreasing, possibly because of less-intensive preparative regimens, better HLA matching, and better definition of occult infections through PCR testing of blood and bronchioalveolar specimens. Diagnostic criteria include signs and symptoms of pneumonia, evidence of nonlobar radiographic infiltrates, and abnormal pulmonary function, all in the absence of documented infectious organisms.[35] Early assessment by bronchioalveolar lavage to rule out infection is important. Traditional therapy has been high-dose methylprednisolone and pulmonary support. Etanercept is a soluble fusion protein that joins the extracellular ligand-binding domain of the TNF-alpha receptor to the Fc region of the IgG1 antibody and that acts by blocking TNF-alpha signaling. The addition of etanercept to steroid therapies has shown promising short-term outcomes (extubation, improved short-term survival) in single-center studies.[36] A large phase II trial of this approach showed promising results, with overall survival rates of 89% at 1 month and 63% at 12 months.[37] Epstein-Barr virus (EBV)-lymphoproliferative disorder EBV infection increases through childhood from approximately 40% in children aged 4 years to more than 80% in teenagers. Patients with a history of previous EBV infection are at risk of EBV reactivation when undergoing HCT procedures that result in intense, prolonged lymphopenia (T-cell-depleted procedures, use of antithymocyte globulin or alemtuzumab, and to a lesser degree, use of cord blood).[38,39,40] Features of EBV reactivation can vary from an isolated increase in EBV titers in the bloodstream as measured by PCR, to an aggressive monoclonal disease with marked lymphadenopathy presenting as lymphoma (lymphoproliferative disorder). Isolated bloodstream reactivation can improve in some cases without therapy as immune function improves; however, lymphoproliferative disorder may require more aggressive therapy. Treatment of EBV-lymphoproliferative disorder has relied on decreasing immune suppression and treatment with chemotherapy agents such as cyclophosphamide. CD20-positive EBV-lymphoproliferative disorder and EBV reactivation have been shown to respond to therapy with the CD20 monoclonal antibody therapy rituximab.[41,42,43] In addition, some centers have found efficacy in treating or preventing this complication with therapeutic or prophylactic EBV-specific cytotoxic T cells.[44] Improved understanding of the risk of EBV reactivation, early monitoring, and aggressive therapy have significantly decreased the risk of mortality from this challenging complication. Acute graft-versus-host disease (GVHD) GVHD is the result of immunologic activation of donor lymphocytes targeting major or minor HLA disparities present in the tissues of a recipient.[45] Acute GVHD usually occurs within the first 3 months posttransplantation, although delayed acute GVHD has been noted in reduced-intensity conditioning and nonmyeloablative approaches, where achieving a high level of full donor chimerism is sometimes delayed. Typically, acute GVHD presents with at least one of three manifestations: skin rash, hyperbilirubinemia, and secretory diarrhea. Acute GVHD is classified by grading the severity of skin, liver, and gastrointestinal involvement and further combining the individual grading of these three areas into an overall stage that is prognostically significant (refer to Tables 6 and 7).[46] Patients with grade III or grade IV acute GVHD are at higher risk of mortality, generally due to organ system damage caused by infections or progressive acute GVHD that is sometimes resistant to therapy. Table 6. Grading and Staging of Acute Graft-Versus-Host Disease (GVHD)a| Stage | Skin | Liver (bilirubin)b | GI/Gut (stool output per day)c |
|---|
| BSA = body surface area; GI = gastrointestinal. | | a Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium consensus, adapted from the modified Glucksberg system. | | b There is no modification of liver staging for other causes of hyperbilirubinemia. | | c For GI staging: Theadult stool output values should be used for patients weighing >50 kg. Use 3-day averages for GI staging based on stool output. If stool and urine are mixed, stool output is presumed to be 50% of total stool/urine mix. | | d If colon or rectal biopsy is positive, but stool output is <500 mL/day (<10 mL/kg/day), then consider as GI stage 0. | | e For stage 4 GI: the termsevere abdominal pain will be defined as having both (a) pain control requiring treatment with opioids or an increased dose in ongoing opioid use; and (b) pain that significantly impacts performance status, as determined by the treating physician. | | 0 | No GVHD rash | <2 mg/dL | Child: <10 mL/kg; adult: <500 mL | | 1 | Maculopapular rash <25% BSA | 2-3 mg/dL | Adult: 500-999 mLd; child: 10-19.9 mL/kg; persistent nausea, vomiting, or anorexia, with a positive upper GI biopsy | | 2 | Maculopapular rash 25%-50% BSA | 3.1-6 mg/dL | Child: 20-30 mL/kg; adult: 1000-1500 mL | | 3 | Maculopapular rash >50% BSA | 6.1-15 mg/dL | Child: >30 mL/kg; adult: >1500 mL | | 4 | Generalized erythroderma plus bullous formation and desquamation >5% BSA | >15 mg/dL | Severe abdominal paine with or without ileus, or grossly bloody stool (regardless of stool volume) | Table 7. Overall Clinical Grade (Based on the Highest Stage Obtained)| GI = gastrointestinal. | | Grade 0: | No stage 1-4 of any organ | | Grade I: | Stage 1-2 skin and no liver or gut involvement | | Grade II: | Stage 3 skin and/or stage 1 liver involvement and/or stage 1 GI | | Grade III: | Stage 0-3 skin, with stage 2-3 liver and/or stage 2-3 GI | | Grade IV: | Stage 4 skin, liver, or GI involvement | Prevention and treatment of acute GVHD Morbidity and mortality from acute GVHD can be reduced through immune suppressive medications given prophylactically or T-cell depletion of grafts, either ex vivo by actual removal of cells from a graft or in vivo with anti-lymphocyte antibodies (antithymocyte globulin or anti-CD52 [alemtuzumab]). Approaches to GVHD prevention in non-T-cell-depleted grafts have included intermittent methotrexate, a calcineurin inhibitor (e.g., cyclosporine or tacrolimus), a combination of a calcineurin inhibitor with methotrexate (currently the most commonly used approach in pediatrics), or various combinations of a calcineurin inhibitor with steroids or mycophenolate mofetil. Non-calcineurin inhibitor approaches (intensive T-cell depletion, posttransplant cyclophosphamide, etc.) have been developed and are becoming more widely used.[47,48] When significant acute GVHD occurs, first-line therapy is generally methylprednisolone.[49] Patients with acute GVHD resistant to this therapy have a poor prognosis, but a good percentage of cases respond to second-line agents (e.g., mycophenolate mofetil, infliximab, pentostatin, sirolimus, or extracorporeal photopheresis).[50] Complete elimination of acute GVHD with intense T-cell depletion approaches has generally resulted in increased relapse, more infectious morbidity, and increased EBV-lymphoproliferative disorder. Because of this, most HCT GVHD prophylaxis is given in an attempt to balance risk by giving sufficient immune suppression to prevent most severe acute GVHD but not completely removing GVHD risk. Chronic GVHD Chronic GVHD is a syndrome that may involve a single organ system or several organ systems, with clinical features resembling autoimmune diseases.[51,52] Chronic GVHD is usually first noted 2 to 12 months after HCT. Traditionally, symptoms occurring more than 100 days after HCT were considered to be chronic GVHD, and symptoms occurring sooner than 100 days post-HCT were considered to be acute GVHD. Because some approaches to HCT can lead to late-onset acute GVHD, and manifestations that are diagnostic for chronic GVHD can occur sooner than 100 days post-HCT, the following three distinct types of chronic GVHD have been described: - Classic chronic GVHD: Occurs with diagnostic and/or distinct features of chronic GVHD (Tables 8-12) after a previous history of resolved acute GVHD.
- Overlap syndrome: An ongoing GVHD process when manifestations diagnostic for chronic GVHD occur while symptoms of acute GVHD persist.
- De novo chronic GVHD: New-onset GVHD generally occurring at least 2 months after transplant, with diagnostic and/or distinct features of chronic GVHD and no history of or features of acute GVHD.
Chronic GVHD occurs in approximately 15% to 30% of children after sibling donor HCT [53] and in 20% to 45% of children after unrelated donor HCT, with higher risk associated with peripheral blood stem cells (PBSCs) and a lower risk with cord blood.[54,55] The tissues that are commonly involved include skin, eyes, mouth, hair, joints, liver, and gastrointestinal tract. Other tissues such as lungs, nails, muscles, urogenital system, and nervous system may be involved. Risk factors for the development of chronic GVHD include the following:[53,56,57] - Patient's age.
- Type of donor.
- Use of PBSCs.
- History of acute GVHD.
- Conditioning regimen.
The diagnosis of chronic GVHD is based on clinical features (at least one diagnostic clinical sign, e.g., poikiloderma) or distinctive manifestations complemented by relevant tests (e.g., dry eye with positive Schirmer test).[58] Tables 8 to 12 list organ manifestations of chronic GVHD with a description of findings that are sufficient to establish the diagnosis of chronic GVHD. Biopsy of affected sites may be needed to confirm the diagnosis.[59] Table 8. Chronic Graft-versus-Host Disease (GVHD) Symptoms in the Skin, Nails, Scalp, and Body Haira| Organ or Site | Diagnosticb | Distinctivec | Other Featuresd | Common (Seen with Both Acute and Chronic GVHD) |
|---|
| a Reprinted fromBiology of Blood and Marrow Transplantation, Volume 11 (Issue 12), Alexandra H. Filipovich, Daniel Weisdorf, Steven Pavletic, Gerard Socie, John R. Wingard, Stephanie J. Lee, Paul Martin, Jason Chien, Donna Przepiorka, Daniel Couriel, Edward W. Cowen, Patricia Dinndorf, Ann Farrell, Robert Hartzman, Jean Henslee-Downey, David Jacobsohn, George McDonald, Barbara Mittleman, J. Douglas Rizzo, Michael Robinson, Mark Schubert, Kirk Schultz, Howard Shulman, Maria Turner, Georgia Vogelsang, Mary E.D. Flowers, National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report, Pages 945-956, Copyright 2005, with permission from American Society for Blood and Marrow Transplantation and Elsevier.[58] | | b Sufficient to establish the diagnosis of chronic GVHD. | | c Seen in chronic GVHD, but insufficient alone to establish a diagnosis of chronic GVHD. | | d Can be acknowledged as part of the chronic GVHD symptomatology if the diagnosis is confirmed. | | e In all cases, infection, drug effects, malignancy, or other causes must be excluded. | | f Diagnosis of chronic GVHD requires biopsy or radiology confirmation (or Schirmer test for eyes). | | Skin | Poikiloderma | Depigmentation | Sweat impairment | Pruritus | | Lichen planus-like features | | Ichthyosis | Erythema | | Sclerotic features | | Keratosis pilaris | Maculopapular rash | | Morphea-like features | | Hypopigmentation | | | Lichen sclerosus-like features | | Hyperpigmentation | | | | | Nails | | Dystrophy | | | | Longitudinal ridging, splitting, or brittle features | | Onycholysis | | Pterygium unguis | | Nail loss (usually symmetric; affects most nails)e | | | | Scalp and body hair | | New onset of scarring or nonscarring scalp alopecia (after recovery from chemoradiotherapy) | Thinning scalp hair, typically patchy, coarse, or dull (not explained by endocrine or other causes) | | | Scaling, papulosquamous lesions | Premature gray hair | Table 9. Chronic Graft-versus-Host Disease (GVHD) Symptoms in the Mouth and GI Tracta| Organ or Site | Diagnosticb | Distinctivec | Other Featuresd | Common (Seen with Both Acute and Chronic GVHD) |
|---|
| ALT = alanine aminotransferase; AST = aspartate aminotransferase; GI = gastrointestinal; ULN = upper limit of normal. | | Refer to Table 8footers for definitions ofa throughe. | | Mouth | Lichen-type features | Xerostomia | | Gingivitis | | Hyperkeratotic plaques | Mucocele | Mucositis | | Restriction of mouth opening from sclerosis | Pseudomembranese | Erythema | | | Mucosal atrophy | Pain | | | Ulcerse | | | | | GI Tract | Esophageal web | | Exocrine pancreatic insufficiency | Anorexia | | Strictures or stenosis in the upper to mid third of the esophaguse | Nausea | | | Vomiting | | | Diarrhea | | | Weight loss | | | Failure to thrive (infants and children) | | | Total bilirubin, alkaline phosphatase >2 × ULNe | | | ALT or AST >2 × ULNe | Table 10. Chronic Graft-versus-Host Disease (GVHD) Symptoms in the Eyesa| Organ or Site | Diagnosticb | Distinctivec | Other Featuresd | Common (Seen with Both Acute and Chronic GVHD) |
|---|
| Refer to Table 8footers for definitions ofa throughf. | | Eyes | | New onset dry, gritty, or painful eyesf | Blepharitis (erythema of the eyelids with edema) | | | Cicatricial conjunctivitis | | Keratoconjunctivitis siccaf | Photophobia | | Confluent areas of punctate keratopathy | Periorbital hyperpigmentation | Table 11. Chronic Graft-versus-Host Disease (GVHD) Symptoms in the Genitaliaa| Organ or Site | Diagnosticb | Distinctivec | Other Featuresd | Common (Seen with Both Acute and Chronic GVHD) |
|---|
| Refer to Table 8footers for definitions ofa throughe. | | Genitalia | Lichen planus-like features | Erosionse | | | | Fissurese | | Vaginal scarring or stenosis | Ulcerse | Table 12. Chronic Graft-versus-Host Disease (GVHD) Symptoms in the Lung, Muscles, Fascia, Joints, Hematopoietic and Immune Systems, and Other Symptomsa| Organ or Site | Diagnosticb | Distinctivec | Other Featuresd | Common (Seen with Both Acute and Chronic GVHD) |
|---|
| AIHA = autoimmune hemolytic anemia; BOOP = bronchiolitis obliterans-organizing pneumonia; ITP = idiopathic thrombocytopenic purpura; PFTs = pulmonary function tests. | | Refer to Table 8footers for definitions ofa throughf. | | Lung | Bronchiolitis obliterans diagnosed with lung biopsy | Bronchiolitis obliterans diagnosed with PFTs and radiologyf | | BOOP | | | | Muscles, fascia, joints | Fasciitis | Myositis or polymyositisf | Edema | | | Muscle cramps | | Arthralgia or arthritis | | | | Hematopoietic and immune | | | Thrombocytopenia | | | Eosinophilia | | Lymphopenia | | Hypo- or hypergammaglobulinemia | | Autoantibodies (AIHA and ITP) | | | | Other | | | Pericardial or pleural effusions | | | Ascites | | Peripheral neuropathy | | Nephrotic syndrome | | Myasthenia gravis | | Cardiac conduction abnormality or cardiomyopathy | Common skin manifestations include alterations in pigmentation, texture, elasticity, and thickness, with papules, plaques, or follicular changes. Patient-reported symptoms include dry skin, itching, limited mobility, rash, sores, or changes in coloring or texture. Generalized scleroderma may lead to severe joint contractures and debility. Associated hair loss and nail changes are common. Other important symptoms that should be assessed include dry eyes and oral changes such as atrophy, ulcers, and lichen planus. In addition, joint stiffness along with restricted range of motion, weight loss, nausea, difficulty swallowing, and diarrhea should be noted. Several factors have been associated with increased risk of nonrelapse mortality in children who develop significant chronic GVHD. Children who received HLA mismatched grafts, PBSCs, who were older than 10 years, or who had platelet counts lower than 100,000/µL at diagnosis of chronic GVHD have an increased risk of nonrelapse mortality. Nonrelapse mortality was 17%, 22%, and 24% at 1, 3, and 5 years, respectively, after diagnosis with chronic GVHD. Many of these children require long-term immune suppression. By 3 years after diagnosis of chronic GVHD, about a third of children had died of either relapse or nonrelapse mortality, a third were off immune suppression, and a third still required some form of immune suppressive therapy.[60] Older literature describes chronic GVHD as either limited or extensive. A National Institutes of Health (NIH) Consensus Workshop in 2006 proposed broadening the description of chronic GVHD to three categories to better predict long-term outcomes.[61] The three NIH grading categories are as follows:[58] - Mild disease: Involving only one or two sites with no significant functional impairment (maximum severity score of 1 on a scale of 0 to 3).
- Moderate disease: Involving either more sites (>2) or associated with higher severity score (maximum score of 2 in any site).
- Severe disease: Indicating major disability (a score of 3 in any site or a lung score of 2).
Thus, high-risk patients include those with severe disease of any site or extensive involvement of multiple sites, especially those with symptomatic lung involvement, skin involvement greater than 50%, platelet count lower than 100,000/µL, poor performance score (<60%), weight loss of more than 15%, chronic diarrhea, progressive onset chronic GVHD, or a history of steroid treatment with more than 0.5 mg of prednisone per kilogram per day for acute GVHD. One study demonstrated a much higher chance of long-term GVHD-free survival and lower treatment-related mortality in children with mild and moderate chronic GVHD than in children with severe chronic GVHD. At 8 years, the probability of continued chronic GVHD in children with mild, moderate, and severe chronic GVHD was 4%, 11%, and 36%, respectively.[62] Treatment of chronic GVHD Steroids remain the cornerstone of chronic GVHD therapy; however, many approaches have been developed to minimize steroid dosing, including use of calcineurin inhibitors.[63] Topical therapy to affected areas is preferred for patients with limited disease.[64] The following agents have been tested with some success: - Mycophenolate mofetil.[65]
- Pentostatin.[66]
- Sirolimus.[67]
- Rituximab.[68]
Other approaches, including extracorporeal photopheresis, have been evaluated and show some efficacy in a percentage of patients.[69] Besides significantly affecting organ function, quality of life, and functional status, infection is the major cause of chronic GVHD-related death. Therefore, all patients with chronic GVHD receive prophylaxis against Pneumocystis jirovecii pneumonia, common encapsulated organisms, and varicella by using agents such as trimethoprim/sulfamethoxazole, penicillin, and acyclovir. While disease progression is the primary cause of death seen in long-term follow-up of hematopoietic stem cell transplantation patients with no chronic GVHD, transplant-related complications account for 70% of the deaths in patients with chronic GVHD.[53] Guidelines concerning ancillary therapy and supportive care of patients with chronic GVHD have been published.[64] Late Mortality After HCT The highest incidence of mortality after HCT occurs in the first 2 years, mostly caused by relapse. A study of late mortality (≥2 years) after HCT showed that about 20% of the 479 patients who were alive at 2 years suffered a late death. Late mortality in the allogeneic group was 15% (median follow-up, 10.0 years; range, 2.0-25.6 years), mainly caused by relapse (65%). A total of 26% of patients suffered a late death after autologous HCT (median follow-up, 6.7 years; range, 2.0-22.2 years).[70] Recurrence of the primary malignancy accounted for 88% of these deaths. In contrast to studies of adult patients, nonrelapse mortality is less common in children, and death caused by chronic GVHD and secondary malignancies is less common. Another study reviewed causes of late mortality after second allogeneic transplantation.[71] Of the children who were alive and relapse free 1 year after second HCT, 55% remained alive at 10 years. The most common cause of mortality at 10 years in this group was relapse (77% of deaths), generally occurring in the first 3 years after transplantation. The cumulative incidence of nonrelapse mortality for this cohort at 10 years was 10%. Chronic GVHD occurred in 43% of children in this study and was the leading cause of nonrelapse mortality. References:
-
Sorror ML, Maris MB, Storb R, et al.: Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106 (8): 2912-9, 2005.
-
ElSawy M, Storer BE, Pulsipher MA, et al.: Multi-centre validation of the prognostic value of the haematopoietic cell transplantation- specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol 170 (4): 574-83, 2015.
-
Sorror ML, Storer BE, Maloney DG, et al.: Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood 111 (1): 446-52, 2008.
-
Sorror ML, Sandmaier BM, Storer BE, et al.: Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol 25 (27): 4246-54, 2007.
-
Sorror M, Storer B, Sandmaier BM, et al.: Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 112 (9): 1992-2001, 2008.
-
Sorror ML, Martin PJ, Storb RF, et al.: Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood 124 (2): 287-95, 2014.
-
Smith AR, Majhail NS, MacMillan ML, et al.: Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood 117 (9): 2728-34, 2011.
-
Wood W, Deal A, Whitley J, et al.: Usefulness of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in predicting outcomes for adolescents and young adults with hematologic malignancies undergoing allogeneic stem cell transplant. Pediatr Blood Cancer 57 (3): 499-505, 2011.
-
Sorror ML, Logan BR, Zhu X, et al.: Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 21 (8): 1479-87, 2015.
-
Burik JH, Freifeld AG: Infection in the severely immunocompromised patient. In: Abeloff MD, Armitage JO, Niederhuber JE, et al.: Clinical Oncology. 3rd ed. Philadelphia, Pa: Elsevier, Churchill Livingstone, 2004, pp 941-56.
-
Antin JH: Immune reconstitution: the major barrier to successful stem cell transplantation. Biol Blood Marrow Transplant 11 (2 Suppl 2): 43-5, 2005.
-
Fry TJ, Mackall CL: Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant 35 (Suppl 1): S53-7, 2005.
-
Wingard JR, Majhail NS, Brazauskas R, et al.: Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29 (16): 2230-9, 2011.
-
Bunin N, Small T, Szabolcs P, et al.: NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: persistent immune deficiency in pediatric transplant survivors. Biol Blood Marrow Transplant 18 (1): 6-15, 2012.
-
Tomblyn M, Chiller T, Einsele H, et al.: Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 15 (10): 1143-238, 2009.
-
Centers for Disease Control and Prevention, Infectious Disease Society of America, American Society of Blood and Marrow Transplantation: Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep 49 (RR-10): 1-125, CE1-7, 2000.
-
Kumar D, Chen MH, Welsh B, et al.: A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis 45 (12): 1576-82, 2007.
-
Reiss U, Cowan M, McMillan A, et al.: Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol 24 (9): 746-50, 2002.
-
Cesaro S, Pillon M, Talenti E, et al.: A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica 90 (10): 1396-404, 2005.
-
Bearman SI: The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 85 (11): 3005-20, 1995.
-
Myers KC, Dandoy C, El-Bietar J, et al.: Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21 (2): 379-81, 2015.
-
Ruutu T, Eriksson B, Remes K, et al.: Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 100 (6): 1977-83, 2002.
-
Myers KC, Lawrence J, Marsh RA, et al.: High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant 19 (3): 500-3, 2013.
-
Richardson PG, Murakami C, Jin Z, et al.: Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood 100 (13): 4337-43, 2002.
-
Corbacioglu S, Kernan N, Lehmann L, et al.: Defibrotide for the treatment of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Expert Rev Hematol 5 (3): 291-302, 2012.
-
Richardson PG, Soiffer RJ, Antin JH, et al.: Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant 16 (7): 1005-17, 2010.
-
Dignan FL, Wynn RF, Hadzic N, et al.: BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol 163 (4): 444-57, 2013.
-
Corbacioglu S, Cesaro S, Faraci M, et al.: Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet 379 (9823): 1301-9, 2012.
-
Ruutu T, Juvonen E, Remberger M, et al.: Improved survival with ursodeoxycholic acid prophylaxis in allogeneic stem cell transplantation: long-term follow-up of a randomized study. Biol Blood Marrow Transplant 20 (1): 135-8, 2014.
-
Jodele S, Licht C, Goebel J, et al.: Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 122 (12): 2003-7, 2013.
-
Cutler C, Henry NL, Magee C, et al.: Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11 (7): 551-7, 2005.
-
Ho VT, Cutler C, Carter S, et al.: Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11 (8): 571-5, 2005.
-
Jodele S, Fukuda T, Vinks A, et al.: Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 20 (4): 518-25, 2014.
-
Kantrow SP, Hackman RC, Boeckh M, et al.: Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation 63 (8): 1079-86, 1997.
-
Clark JG, Hansen JA, Hertz MI, et al.: NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis 147 (6 Pt 1): 1601-6, 1993.
-
Yanik GA, Ho VT, Levine JE, et al.: The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood 112 (8): 3073-81, 2008.
-
Yanik GA, Grupp SA, Pulsipher MA, et al.: TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children's Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 21 (1): 67-73, 2015.
-
Gerritsen EJ, Stam ED, Hermans J, et al.: Risk factors for developing EBV-related B cell lymphoproliferative disorders (BLPD) after non-HLA-identical BMT in children. Bone Marrow Transplant 18 (2): 377-82, 1996.
-
Shapiro RS, McClain K, Frizzera G, et al.: Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 71 (5): 1234-43, 1988.
-
Brunstein CG, Weisdorf DJ, DeFor T, et al.: Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 108 (8): 2874-80, 2006.
-
Blaes AH, Cao Q, Wagner JE, et al.: Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant 16 (2): 287-91, 2010.
-
Kuehnle I, Huls MH, Liu Z, et al.: CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 95 (4): 1502-5, 2000.
-
Styczynski J, Gil L, Tridello G, et al.: Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis 57 (6): 794-802, 2013.
-
Liu Z, Savoldo B, Huls H, et al.: Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res 159: 123-33, 2002.
-
Ferrara JL, Levine JE, Reddy P, et al.: Graft-versus-host disease. Lancet 373 (9674): 1550-61, 2009.
-
Przepiorka D, Weisdorf D, Martin P, et al.: 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15 (6): 825-8, 1995.
-
Kanakry CG, O'Donnell PV, Furlong T, et al.: Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 32 (31): 3497-505, 2014.
-
Bertaina A, Merli P, Rutella S, et al.: HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 124 (5): 822-6, 2014.
-
Jacobsohn DA: Acute graft-versus-host disease in children. Bone Marrow Transplant 41 (2): 215-21, 2008.
-
Deeg HJ: How I treat refractory acute GVHD. Blood 109 (10): 4119-26, 2007.
-
Shlomchik WD, Lee SJ, Couriel D, et al.: Transplantation's greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant 13 (1 Suppl 1): 2-10, 2007.
-
Bolaños-Meade J, Vogelsang GB: Chronic graft-versus-host disease. Curr Pharm Des 14 (20): 1974-86, 2008.
-
Zecca M, Prete A, Rondelli R, et al.: Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood 100 (4): 1192-200, 2002.
-
Eapen M, Logan BR, Confer DL, et al.: Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant 13 (12): 1461-8, 2007.
-
Eapen M, Rubinstein P, Zhang MJ, et al.: Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369 (9577): 1947-54, 2007.
-
Leung W, Ahn H, Rose SR, et al.: A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 86 (4): 215-24, 2007.
-
Arora M, Klein JP, Weisdorf DJ, et al.: Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood 117 (24): 6714-20, 2011.
-
Filipovich AH, Weisdorf D, Pavletic S, et al.: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11 (12): 945-56, 2005.
-
Shulman HM, Kleiner D, Lee SJ, et al.: Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 12 (1): 31-47, 2006.
-
Jacobsohn DA, Arora M, Klein JP, et al.: Risk factors associated with increased nonrelapse mortality and with poor overall survival in children with chronic graft-versus-host disease. Blood 118 (16): 4472-9, 2011.
-
Pavletic SZ, Martin P, Lee SJ, et al.: Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 12 (3): 252-66, 2006.
-
Inagaki J, Moritake H, Nishikawa T, et al.: Long-Term Morbidity and Mortality in Children with Chronic Graft-versus-Host Disease Classified by National Institutes of Health Consensus Criteria after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 21 (11): 1973-80, 2015.
-
Koc S, Leisenring W, Flowers ME, et al.: Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 100 (1): 48-51, 2002.
-
Couriel D, Carpenter PA, Cutler C, et al.: Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 12 (4): 375-96, 2006.
-
Martin PJ, Storer BE, Rowley SD, et al.: Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 113 (21): 5074-82, 2009.
-
Jacobsohn DA, Gilman AL, Rademaker A, et al.: Evaluation of pentostatin in corticosteroid-refractory chronic graft-versus-host disease in children: a Pediatric Blood and Marrow Transplant Consortium study. Blood 114 (20): 4354-60, 2009.
-
Jurado M, Vallejo C, Pérez-Simón JA, et al.: Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant 13 (6): 701-6, 2007.
-
Cutler C, Miklos D, Kim HT, et al.: Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 108 (2): 756-62, 2006.
-
González Vicent M, Ramirez M, Sevilla J, et al.: Analysis of clinical outcome and survival in pediatric patients undergoing extracorporeal photopheresis for the treatment of steroid-refractory GVHD. J Pediatr Hematol Oncol 32 (8): 589-93, 2010.
-
Schechter T, Pole JD, Darmawikarta D, et al.: Late mortality after hematopoietic SCT for a childhood malignancy. Bone Marrow Transplant 48 (10): 1291-5, 2013.
-
Duncan CN, Majhail NS, Brazauskas R, et al.: Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 21 (1): 151-8, 2015.
Late Effects After HCT in ChildrenData from studies of child and adult survivors of hematopoietic cell transplantation (HCT) have shown a significant impact from treatment-related exposures on survival and quality of life.[1] Of those alive at 2 years after HCT, a 9.9-fold increased risk of premature death has been noted.[2] Methodological Challenges Specific to HCT Although the main cause of death in this cohort is from relapse of the primary disease, a sizeable number of these patients die from graft-versus-host disease (GVHD)-related infections, second malignancies, or cardiac or pulmonary issues.[2,3,4,5] In addition, other studies have revealed that up to 40% of HCT survivors experience severe, disabling, and/or life-threatening events or die because of an adverse event associated with cancer treatment.[6,7] Before studies aimed at decreasing the incidence or severity of these effects are initiated, it is important to understand what leads to the development of these complications: - Pretransplant therapy: Pretransplant therapy plays an important role, but the details of significant exposures associated with pre-HCT therapy are not included in many studies.[8]
- Preparative regimen: The transplant preparative regimen itself (e.g., total-body irradiation [TBI], high-dose chemotherapy) has often been studied, but this intense therapy is only a small part of a long course of therapy filled with potential causes of late effects.
- Allogenicity: The effect of allogenicity-differences in major and minor HLA antigens that lead to GVHD, autoimmunity, chronic inflammation, and, sometimes, undetected organ damage-also contributes to these effects.
Individuals differ in their susceptibility to specific organ damage from chemotherapy or in their risk of GVHD on the basis of genetic differences in both the donor and recipient, which modifies the effect of these exposures.[8,9,10] Cardiovascular System Late Effects Although cardiac dysfunction has been studied extensively in non-HCT settings, less is known about the incidence and predictors of congestive heart failure following HCT in childhood. Potentially cardiotoxic exposures unique to HCT include the following:[11] - Conditioning with high-dose chemotherapy, especially cyclophosphamide.
- TBI.
HCT survivors are at increased risk of developing cardiovascular risk factors such as hypertension and diabetes, partly as a result of exposure to TBI and prolonged immunosuppressive therapy following allogeneic HCT, or related to other health conditions (e.g., hypothyroidism or growth hormone deficiency).[7,11] Rates of cardiovascular outcomes were examined among nearly 1,500 transplant survivors (surviving ≥2 years) treated in Seattle from 1985 to 2006. The survivors and a population-based comparison group were matched by age, year, and sex.[12] Survivors experienced increased rates of cardiovascular death (adjusted incidence rate difference, 3.6 per 1,000 person-years [95% confidence interval, 1.7-5.5]) and had an increased cumulative incidence of ischemic heart disease, cardiomyopathy/heart failure, stroke, vascular diseases, and rhythm disorders. Survivors also had an increased cumulative incidence of related conditions that predispose towards more serious cardiovascular disease (i.e., hypertension, renal disease, dyslipidemia, and diabetes). In addition, cardiac function and pre-HCT exposures to chemotherapy and radiation have been shown to have significant impact on post-HCT cardiac function. In evaluating post-HCT patients for long-term issues, it is important to consider levels of pre-HCT anthracycline and chest irradiation.[13] Although more specific work needs to be done to verify this, current evidence suggests that the risk of late-occurring cardiovascular complications after HCT may be largely due to pre-HCT therapeutic exposures, with little additional risk from conditioning-related exposures or GVHD.[14,15] (Refer to the Late Effects of the Cardiovascular System section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Central Nervous System Late Effects Neurocognitive outcomes A preponderance of studies report normal neurodevelopment after HCT, with no evidence of decline.[16,17,18,19,20,21,22,23] Researchers from St. Jude Children's Research Hospital have reported on the largest longitudinal cohort to date, describing remarkable stability in global cognitive function and academic achievement during 5 years of posttransplant follow-up.[19,20,21] This group reported poorer outcome in patients undergoing unrelated donor transplant in those who received TBI and in those who experienced GVHD, but these effects were small compared with the much larger effects seen on the basis of differences in socioeconomic status.[20] Most published studies report similar outcomes. Normal cognitive function and academic achievement were reported in a cohort of 47 patients monitored prospectively through 2 years post-HCT.[23] Stable cognitive function was also noted in a large cohort monitored from pretransplant to 2 years post-HCT.[18] A smaller study reported similar normal functioning and absence of declines over time in HCT survivors.[16] HCT survivors did not differ from their siblings in cognitive and academic function, with the exception that survivors performed better than siblings on measures of perceptual organization.[17] On the basis of the findings to date, it appears that HCT poses low to minimal risk of late cognitive and academic deficits in survivors. A number of studies, however, have reported some decline in cognitive function after HCT.[24,25,26,27,28,29,30] These studies tended to include samples with a high percentage of very young children. One study reported a significant decline in IQ in their cohort at 1 year post-HCT, and these deficits were maintained at 3 years post-HCT.[25,26] Similarly, studies from Sweden have reported deficits in visual-spatial domains and executive functioning in very young children who underwent transplantation with TBI.[28,29] Another study from St. Jude Children's Research Hospital reported that while all children younger than 3 years had a decline in IQ at 1 year after transplant, patients who did not receive TBI during conditioning recovered later. However, patients who received TBI had a significantly lower IQ at 5 years (P = .05) than did those who did not receive TBI.[30] (Refer to the Stem cell transplantation section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Digestive System Late Effects Gastrointestinal, biliary, and pancreatic dysfunction Most gastrointestinal late effects are related to protracted acute GVHD and chronic GVHD (refer to Table 13). (Refer to the Hepatobiliary Complications section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Table 13. Causes of Gastrointestinal (GI), Hepatobiliary, and Pancreatic Problems in Long-Term Transplant Survivorsa| Problem Areas | Common Causes | Less Common Causes |
|---|
| ALT = alanine transaminase; AP = alkaline phosphatase; CMV = cytomegalovirus; GGT = gamma glutamyl transpeptidase; HSV = herpes simplex virus; Mg++ = magnesium; VZV = varicella zoster virus. | | a Reprinted fromBiology of Blood and Marrow Transplantation, Volume 17 (Issue 11), Michael L. Nieder, George B. McDonald, Aiko Kida, Sangeeta Hingorani, Saro H. Armenian, Kenneth R. Cooke, Michael A. Pulsipher , K. Scott Baker, National Cancer Institute-National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on Late Effects After Pediatric Hematopoietic Cell Transplantation: Long-Term Organ Damage and Dysfunction, Pages 1573-1584, Copyright 2011, with permission from American Society for Blood and Marrow Transplantation and Elsevier.[31] | | Esophageal symptoms: heartburn, dysphagia, painful swallowing[32,33,34,35,36,37] | Oral chronic GVHD (mucosal changes, poor dentition, xerostomia) | Chronic GVHD of the esophagus (webs, rings, submucosal fibrosis and strictures, aperistalsis) | | Reflux of gastric fluid | Hypopharyngeal dysmotility (myasthenia gravis, cricopharyngeal incoordination) | | | Squamous > adenocarcinoma | | | Pill esophagitis | | | Infection (fungal, viral) | | | | Upper gut symptoms: anorexia, nausea, vomiting[38,39,40,41,42] | Protracted acute GI GVHD | Secondary adrenal insufficiency | | Activation of latent infection (CMV, HSV, VZV) | Acquisition of infection (enteric viruses, Giardia, cryptosporidia,Haemophilus pylori) | | Medication adverse effects | Gut dysmotility | | | | Mid gut and colonic symptoms: diarrhea and abdominal pain[43,44] | Protracted acute GI GVHD | Acquisition of infection (enteric viruses, bacteria, parasites) | | Activation of latent CMV, VZV | Pancreatic insufficiency | | Drugs (mycophenolate mofetil, Mg++, antibiotics) | Clostridium difficilecolitis | | | Collagen-encased bowel (GVHD) | | | Rare: inflammatory bowel disease, sprue;[44]bile salt malabsorption; disaccharide malabsorption | | | | Liver problems[45,46,47,48,49,50,51,52,53,54,55] | Cholestatic GVHD | Hepatitic GVHD | | Chronic viral hepatitis (B and C) | VZV or HSV hepatitis | | Cirrhosis | Fungal abscess | | Focal nodular hyperplasia | Nodular regenerative hyperplasia | | Nonspecific elevation of liver enzymes in serum (AP, ALT, GGT) | Biliary obstruction | | | Drug-induced liver injury | | | | Biliary and pancreatic problems [56,57,58,59] | Cholecystitis | Pancreatic atrophy/insufficiency | | Common duct stones/sludge | Pancreatitis/edema, stone or sludge related | | Gall bladder sludge (calcium bilirubinate) | Pancreatitis, tacrolimus related | | Gallstones | | As GVHD is controlled and tolerance is developed, most symptoms resolve. Major hepatobiliary concerns include the consequences of viral hepatitis acquired before or during the transplant, biliary stone disease, and focal liver lesions.[45] Viral serology and polymerase chain reaction should be performed to differentiate this from GVHD presenting with hepatocellular injury.[31] Iron overload Iron overload occurs in almost all patients who undergo HCT, especially if the procedure is for a condition associated with transfusion dependence before HCT (e.g., thalassemia, bone marrow failure syndromes) or pre-HCT treatments requiring transfusions after myelotoxic chemotherapy (e.g., acute leukemias). Inflammatory conditions such as GVHD also increase gastrointestinal iron absorption. The effects of iron overload on morbidity post-HCT have not been well studied; however, reducing iron levels after HCT for thalassemia has been shown to improve cardiac function.[60] Non-HCT conditions leading to iron overload can lead to cardiac dysfunction, endocrine disorders (e.g., pituitary insufficiency, hypothyroidism), diabetes, neurocognitive effects, and second malignancies.[31] Although data supporting iron reduction therapies such as phlebotomy or chelation after HCT have not identified specific levels at which iron reduction should be performed, higher levels of ferritin and/or evidence of significant iron overload by liver biopsy or T2-weighted magnetic resonance imaging (MRI) [61] should be addressed by iron reduction therapy.[62] Endocrine System Late Effects Thyroid dysfunction Studies show that rates of thyroid dysfunction in children after myeloablative HCT vary, but larger series report an average incidence of about 30%.[63,64,65,66,67,68,69,70,71,72] A lower incidence in adults (on average, 15%) and a notable increase in incidence in children younger than 10 years undergoing HCT suggest that a developing thyroid gland may be more susceptible to damage.[63,65,69] Pretransplant local thyroid radiation contributes to high rates of thyroid dysfunction in patients with Hodgkin lymphoma.[63] Early studies showed very high rates of thyroid dysfunction after high single-dose fractions of TBI,[73] but traditional fractionated TBI/cyclophosphamide compared with busulfan/cyclophosphamide shows similar rates of thyroid dysfunction, suggesting a role for high-dose chemotherapy in thyroid damage.[66,67,68] Rates of thyroid dysfunction associated with newer combinations of busulfan/fludarabine or reduced-intensity regimens have yet to be reported. (Refer to the Posttransplant thyroid dysfunction section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Of note, higher rates of thyroid dysfunction occur with single-drug versus three-drug GVHD prophylaxis,[74] along with increased rates of thyroid dysfunction after unrelated versus related donor HCT (36% vs. 9%),[64] suggesting a role for alloimmune damage in causing thyroid dysfunction.[68,75] Growth impairment Growth impairment is generally multifactorial. Factors that play a role in failure to achieve expected adult height in young children who have undergone HCT include the following: - Diminished growth hormone level.
- Thyroid dysfunction.
- Disruption of pubertal sex hormone production.
- Steroid therapy.
- Poor nutritional status.
The incidence of growth impairment varies from 20% to 80%, depending on age, risk factors, and the definition of growth impairment used by reporting groups.[70,71,76,77,78,79] Risk factors include the following:[66,67,77,80] - TBI.
- Cranial irradiation.
- Younger age.
- Undergoing HCT for acute lymphoblastic leukemia.
- HCT occurring during a pubertal growth spurt.[81]
Patients younger than 10 years at the time of HCT are at highest risk of growth impairment, but also respond best to growth hormone replacement therapy. Early screening and referral of patients with signs of growth impairment to endocrinology specialists can result in significant restoration of height in younger children.[79] (Refer to the Growth hormone deficiency (GHD) section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Abnormal body composition/metabolic syndrome Adult survivors after allogeneic HCT have a risk of premature cardiovascular-related death that is increased 2.3-fold compared with the general population.[82,83] The exact etiology of cardiovascular risk and subsequent death is largely unknown, although development of metabolic syndrome (a constellation of central obesity, insulin resistance, glucose intolerance, dyslipidemia, and hypertension), especially insulin resistance, as a consequence of HCT has been suggested.[84,85,86] In studies of conventionally treated leukemia survivors compared with those who underwent HCT, transplant survivors are significantly more likely to manifest metabolic syndrome or multiple adverse cardiac risk factors, including central adiposity, hypertension, insulin resistance, and dyslipidemia.[31,87,88] The concern over time is that survivors who develop metabolic syndrome after HCT will be at higher risk of developing significant cardiovascular-related events and/or premature death from cardiovascular-related causes. (Refer to the Metabolic Syndrome section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Sarcopenic obesity The association of obesity with diabetes and cardiovascular disease risk in the general population is well established, but obesity as determined by body mass index (BMI) is uncommon in long-term survivors after HCT.[88] However, despite having a normal BMI, HCT survivors develop significantly altered body composition that results in both an increase in total percent fat mass and a reduction in lean body mass. This finding is termed sarcopenic obesity and results in a loss of myocyte insulin receptors and an increase in adipocyte insulin receptors; the latter are less efficient in binding insulin and clearing glucose, ultimately contributing to insulin resistance.[89,90,91] Preliminary data from 119 children and young adults and 81 healthy sibling controls found that HCT survivors had significantly lower weight but no differences in BMI or waist circumference when compared with siblings.[92] HCT survivors had a significantly higher percent fat mass and lower lean body mass than did controls. HCT survivors were significantly more insulin resistant than were controls, and they also had a higher incidence of other cardiovascular risk factors such as total cholesterol, low-density lipoprotein cholesterol, and triglycerides. Of note, these differences were found only in patients who had received TBI as part of their transplant conditioning regimen. Musculoskeletal System Late Effects Low bone mineral density A limited number of studies have addressed low bone mineral density after HCT in children.[93,94,95,96,97,98,99] A significant portion of children experienced reduction in total-body bone mineral density or lumbar Z-scores showing osteopenia (18%-33%) or osteoporosis (6%-21%). Although general risk factors have been described (female gender, inactivity, poor nutritional status, white or Asian ethnicity, family history, TBI, craniospinal irradiation, corticosteroid therapy, GVHD, cyclosporine, and endocrine deficiencies [e.g., growth hormone deficiency, hypogonadism]), most reported populations have been too small to perform multivariate analysis to test the relative importance of each of these factors.[100,101,102,103,104,105,106,107,108,109,110] Some studies in adults have shown improvement over time in low bone mineral density after HCT;[98,111,112] however, this has yet to be shown in children. Treatment for children has generally included a multifactorial approach, with vitamin D and calcium supplementation, minimization of corticosteroid therapy, weight-bearing exercise, and resolution of other endocrine problems. The role of bisphosphonate therapy in children with this condition is unclear. (Refer to the Osteoporosis/fractures section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Osteonecrosis Reported incidence of osteonecrosis in children after HCT was 1% to 14%; however, these studies were retrospective and underestimated actual incidence because patients may be asymptomatic early in the course. Two prospective studies have shown an incidence of 30% to 44% with routine MRI screening of possible target joints.[97,113] Osteonecrosis generally occurs within 3 years after HCT, with a median onset of about 1 year. The most common locations include knees (30%-40%), hips (19%-24%), and shoulders (9%). Most patients experience osteonecrosis in two or more joints.[73,114,115,116] In one prospective report, risk factors by multivariate analysis included age (markedly increased in children older than 10 years; odds ratio, 7.4) and presence of osteonecrosis at the time of transplant. It is important to note that pre-HCT factors such as corticosteroid exposure are very important in determining patient risk. In this study, 14 of 44 children who developed osteonecrosis had the disease before HCT.[113] A Center for International Blood and Marrow Transplant Research (CIBMTR) retrospective nested control study of 160 cases and 478 control children suggested older age (>5 years), female gender, and the presence of chronic GVHD as risk factors for osteonecrosis.[117] Treatment has generally consisted of minimization of corticosteroid therapy and surgical joint replacement. Most patients are not diagnosed until they present with symptoms. Of note, in one study of 44 patients with osteonecrosis lesions in whom routine yearly MRI was performed, four resolved completely, and two had resolution of one of multiply involved joints.[113] The observation that some lesions can heal over time suggests caution in the surgical management of asymptomatic lesions. (Refer to the Osteonecrosis section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Reproductive System Late Effects Pubertal development Delayed, absent, or incomplete pubertal development occurs commonly after HCT. Two studies showed pubertal delay or failure in 16% of female children who received cyclophosphamide alone, 72% of those who received busulfan/cyclophosphamide, and 57% of those who underwent fractionated TBI. In males, incomplete pubertal development or failure was noted in 14% of those who received cyclophosphamide alone, 48% of those who received busulfan/cyclophosphamide, and 58% of those who underwent TBI.[72,118] Boys receiving more than 24 Gy of radiation to the testicles developed azoospermia and also experienced failure of testosterone production, requiring supplementation to develop secondary sexual characteristics.[119] Fertility Women Pretransplant and transplant cyclophosphamide exposure is the best studied agent affecting fertility. Postpubertal women younger than 30 years can tolerate up to 20 g/m2 of cyclophosphamide and have preserved ovarian function; prepubertal females can tolerate as much as 25g/m2 to 30 g/m2. Although the additional effect added by pretransplant exposures to cyclophosphamide and other agents has not been specifically quantitated in studies, these exposures plus transplant-related chemotherapy and radiation therapy lead to ovarian failure in 65% to 84% of females undergoing myeloablative HCT.[120,121,122,123] The use of cyclophosphamide, busulfan, and TBI as part of the preparative regimen are associated with worse ovarian function. Younger age at the time of HCT is associated with a higher chance of menarche and ovulation.[124,125] (Refer to the Ovarian function after HSCT section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Studies of pregnancy are challenging because data seldom indicate whether individuals are trying to conceive. Nonetheless, a large study of pregnancy in pediatric and adult survivors of myeloablative transplantation demonstrated conception in 32 of 708 patients (4.5%).[120] Of those trying to conceive, patients exposed to cyclophosphamide alone (total dose 6.7 g/m2 with no pretransplant exposure) had the best chance of conception (56 of 103, 54%), while those receiving myeloablative busulfan/cyclophosphamide (0 of 73, 0%) or TBI (7 of 532, 1.3%) had much lower rates of conception. Men The ability of men to produce functional sperm decreases with exposure to higher doses and specific types of chemotherapy. Most men will become azoospermic at a cyclophosphamide dose of 300 mg/kg.[126] After HCT, 48% to 85% will experience gonadal failure.[120,126,127] One study showed that men who received cyclophosphamide conceived only 24% of the time, compared with 6.5% of men who received busulfan/cyclophosphamide and 1.3% of those who underwent TBI.[120] (Refer to the Testicular function after HSCT section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Effect of reduced toxicity/reduced intensity/nonmyeloablative regimens On the basis of clear evidence of dose effect and the lowered gonadotoxicity of some reduced-toxicity chemotherapy regimens, the use of reduced intensity/toxicity and nonmyeloablative regimens will likely lead to a higher chance of preserved fertility after HCT. Because the use of these regimens is relatively new and mostly confined to older or sicker patients, most reports have consisted of single cases. Registry reports are beginning to describe pregnancies after these procedures.[123] In addition, a single-center study compared myeloablative busulfan/cyclophosphamide with reduced-intensity fludarabine/melphalan.[128][Level of evidence: 3iiiC] Spontaneous puberty occurred in 56% of girls and 89% of boys after busulfan/cyclophosphamide, whereas 90% of girls and all of the boys in the fludarabine/melphalan group entered puberty spontaneously (P = .012). Significantly more girls (61%) conditioned with busulfan/cyclophosphamide required hormone replacement than did in the fludarabine/melphalan group (10.5%; P = .012). In boys, no difference was noted between the two conditioning groups in time to follicle-stimulating hormone elevation (median 4 years in the fludarabine/melphalan group vs. 6 years in the busulfan/cyclophosphamide group). While the two regimens have similar effects on the testis, ovarian function seems to be better preserved in girls undergoing stem cell transplantation with reduced-intensity conditioning approaches. Respiratory System Late Effects Chronic pulmonary dysfunction Two forms of chronic pulmonary dysfunction are observed after HCT:[129,130,131,132,133,134] - Obstructive lung disease.
- Restrictive lung disease.
The incidence of both forms of lung toxicity can range from 10% to 40%, depending on donor source, the time interval after HCT, definition applied, and presence of chronic GVHD. In both conditions, collagen deposition and the development of fibrosis in either the interstitial space (restrictive lung disease) or the peribronchiolar space (obstructive lung disease) are believed to underlie the pathology.[135] The most common form of obstructive lung disease post-allogeneic HCT is bronchiolitis obliterans.[131,134,136,137] This condition is an inflammatory process resulting in bronchiolar obliteration, fibrosis, and progressive obstructive lung disease.[129] Historically, the term bronchiolitis obliterans has been used to describe chronic GVHD of the lung and begins 6 to 20 months after HCT. Pulmonary function tests show obstructive lung disease with general preservation of forced vital capacity (FVC), reductions in forced expiratory volume in 1 second (FEV1), and associated decreases in the FEV1/FVC ratio with or without significant declines in the diffusion capacity of the lung for carbon monoxide (DLCO). Risk factors for bronchiolitis obliterans include the following:[129,136] - Lower pretransplant FEV1/FVC values.
- Concomitant pulmonary infections.
- Chronic aspiration.
- Acute and chronic GVHD.
- Older recipient age.
- Use of mismatched donors.
- High-dose (vs. reduced-intensity) conditioning.
The clinical course of bronchiolitis obliterans is variable, but patients frequently develop progressive and debilitating respiratory failure despite the initiation of enhanced immunosuppression. Restrictive lung disease is defined by reductions in FVC, total lung capacity (TLC), and DLCO. In contrast to obstructive lung disease, the FEV1/FVC ratio is maintained near 100%. Restrictive lung disease is common after HCT and has been reported in 25% to 45% of patients by day 100.[129] Importantly, declines in TLC or FVC occurring at 100 days and 1 year after HCT are associated with an increase in nonrelapse mortality. Early reports suggested that the incidence of restrictive lung disease increases with advancing recipient age, but subsequent studies have revealed significant restrictive lung disease in children receiving HCT.[138] The most recognizable form of restrictive lung disease is bronchiolitis obliterans organizing pneumonia. Clinical features include dry cough, shortness of breath, and fever. Radiographic findings show diffuse, peripheral, fluffy infiltrates consistent with airspace consolidation. Although reported in fewer than 10% of HCT recipients, the development of bronchiolitis obliterans organizing pneumonia is strongly associated with previous acute and chronic GVHD.[135] Standard treatment for obstructive lung disease combines enhanced immunosuppression with supportive care, including antimicrobial prophylaxis, bronchodilator therapy, and supplemental oxygen, when indicated. Unfortunately, the response in patients with restrictive lung disease to multiple agents such as corticosteroids, cyclosporine, tacrolimus, and azathioprine is limited.[139] The potential role for tumor necrosis factor-alpha in the pathogenesis of both obstructive and restrictive lung disease suggests that neutralizing agents such as etanercept may have promise.[140] (Refer to the Respiratory complications associated with HSCT section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.) Urinary System Late Effects Renal disease Chronic kidney disease is frequently diagnosed after transplant. There are many clinical forms of chronic kidney disease, but the most commonly described ones include thrombotic microangiopathy, nephrotic syndrome, calcineurin inhibitor toxicity, acute kidney injury, and GVHD-related chronic kidney disease. Various risk factors associated with the development of chronic kidney disease have been described; however, recent studies suggest that acute and chronic GVHD may be a proximal cause of renal injury.[31] In a systematic review of 9,317 adults and children from 28 cohorts who underwent HCT, approximately 16.6% (range, 3.6% to 89%) of patients developed chronic kidney disease, defined as a decrease in estimated glomerular filtration rate of at least 24.5 mL/min/1.73 m2 within the first year after transplant.[141] The cumulative incidence of chronic kidney disease developing approximately 5 years after transplant ranges from 4.4% to 44.3%, depending on the type of transplant and stage of chronic kidney disease.[142,143] Mortality rates among patients with chronic kidney disease in this setting are higher than those in transplant recipients who retain normal renal function, even when studies have controlled for comorbidities.[144] It is important to aggressively treat hypertension in patients post-HCT, especially in those treated with prolonged courses of calcineurin inhibitors. Whether post-HCT patients with albuminuria and hypertension benefit from treatment with angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers requires further study, but careful control of hypertension with captopril, an ACE inhibitor, did show a benefit in a small study.[145] Quality of Life Health-related quality of life (HRQL) HRQL is a multidimensional construct, incorporating a subjective appraisal of one's functioning and well-being, with reference to the impact of the health issues on overall quality of life.[146,147]. Many studies have shown that HRQL varies according to the following:[148] - Time after HCT: HRQL is worse with more recent HCT.
- Transplant type: Unrelated donor HCT has worse HRQL than does autologous or allogeneic-related HCT.
- Presence or absence of HCT-related sequelae: HRQL is worse with chronic GVHD.
Pre-HCT factors, such as family cohesion and the child's adaptive functioning, have been shown to affect HRQL.[149] Several groups have also identified the importance of pre-HCT parenting stress on parental ratings of children's HRQL post-HCT.[149,150,151,152,153] A report of the trajectories of HRQL over the 12 months after HCT noted that the poorest HRQL was seen at 3 months post-HCT, with steady improvement thereafter. Recipients of unrelated donor transplants had the steepest declines in HRQL from baseline to 3 months. Another study reported that compromised emotional functioning, high levels of worry, and reduced communication during the acute recovery period had a negative impact on HRQL at 1-year post-HCT.[154] Longitudinal studies identified an association of the following additional baseline risk factors with the trajectory of HRQL following HCT: - Child's age (older children, worse HRQL).[149,155,156]
- Child's gender (females, worse HRQL).[156]
- Rater (mothers report lower HRQL than do fathers; parents report lower HRQL than do children).[157,158]
- Concordance by primary language or by gender of the raters (concordant pairs, higher HRQL).[159]
- Parental emotional distress (greater parental distress, worse HRQL).[155]
- Child's race (African American children, better HRQL).[156]
A report on the impact of specific HCT complications on children's HRQL indicated that HRQL was worse among children with severe end-organ toxicity, systemic infection, or GVHD.[150] Cross-sectional studies report that the HRQL among pediatric HCT survivors of 5 years or more is reasonably good, although psychological, cognitive, or physical problems appear to negatively influence HRQL. Female gender, causal diagnosis for HCT (acute myelogenous leukemia, worse HRQL), and intensity of pre-HCT therapy were all identified as affecting HRQL post-HCT.[160,161] Finally, another cross-sectional study of children 5 to 10 years post-HCT cautioned that parental concerns about the child's vulnerability may induce overprotective parenting.[153] Functional outcomes Physician-reported physical performance Clinician reports of long-term disability among childhood HCT survivors suggest that the prevalence and severity of functional loss is low. A study from the European Group for Blood and Marrow Transplantation used the Karnofsky performance scale to report outcomes among 647 HCT survivors (surviving ≥5 years).[162] In this cohort, 40% of survivors were younger than 18 years when transplanted; only 19% had Karnofsky scores lower than 100. Seven percent had scores lower than 80, defined as the inability to work. Similar low rates of clinician-graded poor functional outcome were reported by two other groups.[160,163] Among 50 survivors of childhood allogeneic HCT treated at the City of Hope National Medical Center and Stanford University Hospital, all had Karnofsky scores of 90 or 100.[163] Among 73 young adults (mean age, 26 years) treated at the Karolinska University Hospital, the median Karnofsky score at 10 years post-HCT was 90.[160] Self-reported physical performance Self-reported and proxy data among survivors of childhood HCT indicate similar low rates of functional loss. One study evaluated 22 survivors of childhood allogeneic HCT (mean age at HCT, 11 years; mean age at questionnaire, 25 years) and reported no differences between survivors' scores and population-expected values on standardized physical performance scales.[164] Another study compared a group of survivors transplanted for childhood leukemia (n = 142) with a group of childhood leukemia survivors treated with chemotherapy alone (n = 288).[165] There were no differences between the groups on the physical function and leisure scales using multiple standardized measures Conversely, in the Bone Marrow Transplant Survivors Study (BMTSS), among 235 survivors of childhood HCT, 17% reported long-term physical performance limitations compared with 8.7% of a sibling comparison group.[166] Additionally, a Seattle study evaluated physical function in 214 young adults (median age at questionnaire, 28.7 years; 118 males) who were transplanted at a median age of 11.9 years. When compared with age- and sex-matched controls, the HCT survivors in this cohort scored one-half standard deviation lower on the physical function, role physical, and physical component summary subscales of the SF-36, a quality-of-life measure.[161] Finally, a Swedish study also identified lower self-reported physical health among 73 young adult (median age, 26 years) HCT survivors who were a median of 10 years from transplant. HCT survivors scored significantly below population normative values on physical functioning (90.2 for HCT survivors vs. 95.3 for population), satisfaction with physical health (66.0 for HCT survivors vs. 78.7 for population), and role limitation due to physical health (72.7 for HCT survivors vs. 84.9 for population).[160] Measured physical performance Objective measurements of function in the pediatric HCT patient and survivor population hints that loss of physical capacity may be a bigger problem than revealed in studies that rely on either clinician or self-report data. Studies measuring cardiopulmonary fitness have observed the following: - One study used exercise capacity with cycle ergometry in a group of 20 children and young adults before HCT, 31 patients at 1 year post-HCT, and 70 healthy controls.[167] Average peak oxygen consumption was 21 mL/kg/min in the pre-HCT group, 24 mL/kg/min in the post-HCT group, and 34 mL/kg/min in the healthy controls. Among the HCT survivors, 62% of those with cancer diagnoses scored in the lowest fifth percentile for peak oxygen consumption, compared with healthy controls.
- Another study examined exercise capacity with a Bruce treadmill protocol in 31 survivors of pediatric HCT. In this cohort, 25.8% of HCT survivors had exercise capacities in the 70% to 79% of predicted category, and 41.9% had exercise capacities in the lower than 70% of predicted category.[168]
- In a third study of exercise capacity among 33 HCT survivors who underwent transplantation at a mean age of 11.3 years, at the 5-year post-HCT time point, only 4 of 33 survivors scored above the 75th percentile on a serial cycle ergometry test.[169]
Predictors of poor physical performance In the BMTSS, associations were found between chronic GVHD, cardiac conditions, immune suppression, or treatment for a second malignant neoplasm and poor physical performance outcomes.[170] In the a study from the Fred Hutchison Cancer Research Center, poor performance was associated with myeloid disease.[161] Published Guidelines for Long-term Follow-up A number of organizations have put forward consensus guidelines for follow-up for late effects after HCT. The CIBMTR along with the American Society of Blood and Marrow Transplant (ASBMT) and in cooperation with five other international transplant groups, published consensus recommendations for screening and preventive practices for long-term survivors of HCT.[171] Although some pediatric-specific challenges are addressed in these guidelines, many important pediatric issues are not. Some of these issues have been partially covered by general guidelines published by the Children's Oncology Group (COG) and other children's cancer groups (United Kingdom, Scotland, and Netherlands). To address the lack of detailed pediatric-specific late effects data and guidelines for long-term follow-up after HCT, the Pediatric Blood and Marrow Transplant Consortium (PBMTC) published six detailed papers outlining existing data and summarizing recommendations from key groups (CIBMTR/ASBMT, COG, and the United Kingdom), along with expert recommendations for pediatric-specific issues.[8,31,62,172,173,174] Although international efforts at further standardization and harmonization of pediatric-specific follow-up guidelines are under way, the PBMTC summary and guideline recommendations provide the most current outline for monitoring children for late effects after HCT.[62] References:
-
Sun CL, Francisco L, Kawashima T, et al.: Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood 116 (17): 3129-39; quiz 3377, 2010.
-
Bhatia S, Francisco L, Carter A, et al.: Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 110 (10): 3784-92, 2007.
-
Bhatia S, Robison LL, Francisco L, et al.: Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood 105 (11): 4215-22, 2005.
-
Gooley TA, Chien JW, Pergam SA, et al.: Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 363 (22): 2091-101, 2010.
-
Martin PJ, Counts GW Jr, Appelbaum FR, et al.: Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol 28 (6): 1011-6, 2010.
-
Geenen MM, Cardous-Ubbink MC, Kremer LC, et al.: Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297 (24): 2705-15, 2007.
-
Oeffinger KC, Mertens AC, Sklar CA, et al.: Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355 (15): 1572-82, 2006.
-
Bhatia S, Davies SM, Scott Baker K, et al.: NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: etiology and pathogenesis of late effects after HCT performed in childhood--methodologic challenges. Biol Blood Marrow Transplant 17 (10): 1428-35, 2011.
-
Behar E, Chao NJ, Hiraki DD, et al.: Polymorphism of adhesion molecule CD31 and its role in acute graft-versus-host disease. N Engl J Med 334 (5): 286-91, 1996.
-
Goodman RS, Ewing J, Evans PC, et al.: Donor CD31 genotype and its association with acute graft-versus-host disease in HLA identical sibling stem cell transplantation. Bone Marrow Transplant 36 (2): 151-6, 2005.
-
van Dalen EC, van der Pal HJ, Kok WE, et al.: Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer 42 (18): 3191-8, 2006.
-
Chow EJ, Mueller BA, Baker KS, et al.: Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med 155 (1): 21-32, 2011.
-
Armenian SH, Sun CL, Francisco L, et al.: Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol 26 (34): 5537-43, 2008.
-
Armenian SH, Sun CL, Mills G, et al.: Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant 16 (8): 1138-44, 2010.
-
Armenian SH, Sun CL, Kawashima T, et al.: Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 118 (5): 1413-20, 2011.
-
Arvidson J, Kihlgren M, Hall C, et al.: Neuropsychological functioning after treatment for hematological malignancies in childhood, including autologous bone marrow transplantation. Pediatr Hematol Oncol 16 (1): 9-21, 1999 Jan-Feb.
-
Barrera M, Atenafu E: Cognitive, educational, psychosocial adjustment and quality of life of children who survive hematopoietic SCT and their siblings. Bone Marrow Transplant 42 (1): 15-21, 2008.
-
Kupst MJ, Penati B, Debban B, et al.: Cognitive and psychosocial functioning of pediatric hematopoietic stem cell transplant patients: a prospective longitudinal study. Bone Marrow Transplant 30 (9): 609-17, 2002.
-
Phipps S, Brenner M, Heslop H, et al.: Psychological effects of bone marrow transplantation on children and adolescents: preliminary report of a longitudinal study. Bone Marrow Transplant 15 (6): 829-35, 1995.
-
Phipps S, Rai SN, Leung WH, et al.: Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol 26 (12): 2027-33, 2008.
-
Phipps S, Dunavant M, Srivastava DK, et al.: Cognitive and academic functioning in survivors of pediatric bone marrow transplantation. J Clin Oncol 18 (5): 1004-11, 2000.
-
Cognitive functioning after BMT. In: Pot-Mees CC: The Psychological Effects of Bone Marrow Transplantation in Children. The Netherlands: Eburon Delft, 1989, pp 96-103.
-
Simms S, Kazak AE, Golomb V, et al.: Cognitive, behavioral, and social outcome in survivors of childhood stem cell transplantation. J Pediatr Hematol Oncol 24 (2): 115-9, 2002.
-
Cool VA: Long-term neuropsychological risks in pediatric bone marrow transplant: what do we know? Bone Marrow Transplant 18 (Suppl 3): S45-9, 1996.
-
Kramer JH, Crittenden MR, DeSantes K, et al.: Cognitive and adaptive behavior 1 and 3 years following bone marrow transplantation. Bone Marrow Transplant 19 (6): 607-13, 1997.
-
Kramer JH, Crittenden MR, Halberg FE, et al.: A prospective study of cognitive functioning following low-dose cranial radiation for bone marrow transplantation. Pediatrics 90 (3): 447-50, 1992.
-
Shah AJ, Epport K, Azen C, et al.: Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Pediatr Hematol Oncol 30 (6): 411-8, 2008.
-
Smedler AC, Bolme P: Neuropsychological deficits in very young bone marrow transplant recipients. Acta Paediatr 84 (4): 429-33, 1995.
-
Smedler AC, Winiarski J: Neuropsychological outcome in very young hematopoietic SCT recipients in relation to pretransplant conditioning. Bone Marrow Transplant 42 (8): 515-22, 2008.
-
Willard VW, Leung W, Huang Q, et al.: Cognitive outcome after pediatric stem-cell transplantation: impact of age and total-body irradiation. J Clin Oncol 32 (35): 3982-8, 2014.
-
Nieder ML, McDonald GB, Kida A, et al.: National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant 17 (11): 1573-84, 2011.
-
Mackey JR, Desai S, Larratt L, et al.: Myasthenia gravis in association with allogeneic bone marrow transplantation: clinical observations, therapeutic implications and review of literature. Bone Marrow Transplant 19 (9): 939-42, 1997.
-
Shimada K, Yokozawa T, Atsuta Y, et al.: Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant 36 (2): 115-21, 2005.
-
McDonald GB, Sullivan KM, Schuffler MD, et al.: Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology 80 (5 pt 1): 914-21, 1981.
-
McDonald GB, Sullivan KM, Plumley TF: Radiographic features of esophageal involvement in chronic graft-vs.-host disease. AJR Am J Roentgenol 142 (3): 501-6, 1984.
-
Schima W, Pokieser P, Forstinger C, et al.: Videofluoroscopy of the pharynx and esophagus in chronic graft-versus-host disease. Abdom Imaging 19 (3): 191-4, 1994 May-Jun.
-
Minocha A, Mandanas RA, Kida M, et al.: Bullous esophagitis due to chronic graft-versus-host disease. Am J Gastroenterol 92 (3): 529-30, 1997.
-
Patey-Mariaud de Serre N, Reijasse D, Verkarre V, et al.: Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant 29 (3): 223-30, 2002.
-
Akpek G, Chinratanalab W, Lee LA, et al.: Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant 9 (1): 46-51, 2003.
-
Filipovich AH, Weisdorf D, Pavletic S, et al.: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11 (12): 945-56, 2005.
-
McDonald GB, Bouvier M, Hockenbery DM, et al.: Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology 115 (1): 28-35, 1998.
-
Hockenbery DM, Cruickshank S, Rodell TC, et al.: A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood 109 (10): 4557-63, 2007.
-
Iyer RV, Hahn T, Roy HN, et al.: Long-term use of oral beclomethasone dipropionate for the treatment of gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 11 (8): 587-92, 2005.
-
Borgaonkar MR, Duggan PR, Adams G: Differing clinical manifestations of celiac disease transmitted by bone marrow transplantation. Dig Dis Sci 51 (1): 210-2, 2006.
-
McDonald GB: Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology 51 (4): 1450-60, 2010.
-
Sullivan KM, Shulman HM, Storb R, et al.: Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 57 (2): 267-76, 1981.
-
Tomás JF, Pinilla I, García-Buey ML, et al.: Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant 26 (6): 649-55, 2000.
-
Strasser SI, Shulman HM, Flowers ME, et al.: Chronic graft-versus-host disease of the liver: presentation as an acute hepatitis. Hepatology 32 (6): 1265-71, 2000.
-
Malik AH, Collins RH Jr, Saboorian MH, et al.: Chronic graft-versus-host disease after hematopoietic cell transplantation presenting as an acute hepatitis. Am J Gastroenterol 96 (2): 588-90, 2001.
-
Akpek G, Boitnott JK, Lee LA, et al.: Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood 100 (12): 3903-7, 2002.
-
Shulman HM, Sharma P, Amos D, et al.: A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology 8 (3): 463-70, 1988 May-Jun.
-
Strasser SI, Myerson D, Spurgeon CL, et al.: Hepatitis C virus infection and bone marrow transplantation: a cohort study with 10-year follow-up. Hepatology 29 (6): 1893-9, 1999.
-
Ljungman P, Johansson N, Aschan J, et al.: Long-term effects of hepatitis C virus infection in allogeneic bone marrow transplant recipients. Blood 86 (4): 1614-8, 1995.
-
Strasser SI, Sullivan KM, Myerson D, et al.: Cirrhosis of the liver in long-term marrow transplant survivors. Blood 93 (10): 3259-66, 1999.
-
Peffault de Latour R, Lévy V, Asselah T, et al.: Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood 103 (5): 1618-24, 2004.
-
Ko CW, Murakami C, Sekijima JH, et al.: Chemical composition of gallbladder sludge in patients after marrow transplantation. Am J Gastroenterol 91 (6): 1207-10, 1996.
-
Akpek G, Valladares JL, Lee L, et al.: Pancreatic insufficiency in patients with chronic graft-versus-host disease. Bone Marrow Transplant 27 (2): 163-6, 2001.
-
Maringhini A, Gertz MA, DiMagno EP: Exocrine pancreatic insufficiency after allogeneic bone marrow transplantation. Int J Pancreatol 17 (3): 243-7, 1995.
-
Radu B, Allez M, Gornet JM, et al.: Chronic diarrhoea after allogenic bone marrow transplantation. Gut 54 (1): 161, 174, 2005.
-
Mariotti E, Angelucci E, Agostini A, et al.: Evaluation of cardiac status in iron-loaded thalassaemia patients following bone marrow transplantation: improvement in cardiac function during reduction in body iron burden. Br J Haematol 103 (4): 916-21, 1998.
-
Angelucci E, Muretto P, Lucarelli G, et al.: Treatment of iron overload in the "ex-thalassemic". Report from the phlebotomy program. Ann N Y Acad Sci 850: 288-93, 1998.
-
Pulsipher MA, Skinner R, McDonald GB, et al.: National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biol Blood Marrow Transplant 18 (3): 334-47, 2012.
-
Sanders JE, Hoffmeister PA, Woolfrey AE, et al.: Thyroid function following hematopoietic cell transplantation in children: 30 years' experience. Blood 113 (2): 306-8, 2009.
-
Bailey HK, Kappy MS, Giller RH, et al.: Time-course and risk factors of hypothyroidism following allogeneic hematopoietic stem cell transplantation (HSCT) in children conditioned with fractionated total body irradiation. Pediatr Blood Cancer 51 (3): 405-9, 2008.
-
Ishiguro H, Yasuda Y, Tomita Y, et al.: Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab 89 (12): 5981-6, 2004.
-
Michel G, Socié G, Gebhard F, et al.: Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation--a report from the Société Française de Greffe de Moelle. J Clin Oncol 15 (6): 2238-46, 1997.
-
Afify Z, Shaw PJ, Clavano-Harding A, et al.: Growth and endocrine function in children with acute myeloid leukaemia after bone marrow transplantation using busulfan/cyclophosphamide. Bone Marrow Transplant 25 (10): 1087-92, 2000.
-
Slatter MA, Gennery AR, Cheetham TD, et al.: Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant 33 (9): 949-53, 2004.
-
Berger C, Le-Gallo B, Donadieu J, et al.: Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant 35 (10): 991-5, 2005.
-
Leung W, Ahn H, Rose SR, et al.: A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 86 (4): 215-24, 2007.
-
Dvorak CC, Wright NB, Wong WB, et al.: Safety of hematopoietic stem cell transplantation in children less than three years of age. Pediatr Hematol Oncol 25 (8): 705-22, 2008.
-
Sanders JE, Woolfrey AE, Carpenter PA, et al.: Late effects among pediatric patients followed for nearly 4 decades after transplantation for severe aplastic anemia. Blood 118 (5): 1421-8, 2011.
-
Socié G, Salooja N, Cohen A, et al.: Nonmalignant late effects after allogeneic stem cell transplantation. Blood 101 (9): 3373-85, 2003.
-
Katsanis E, Shapiro RS, Robison LL, et al.: Thyroid dysfunction following bone marrow transplantation: long-term follow-up of 80 pediatric patients. Bone Marrow Transplant 5 (5): 335-40, 1990.
-
Savani BN, Koklanaris EK, Le Q, et al.: Prolonged chronic graft-versus-host disease is a risk factor for thyroid failure in long-term survivors after matched sibling donor stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant 15 (3): 377-81, 2009.
-
Huma Z, Boulad F, Black P, et al.: Growth in children after bone marrow transplantation for acute leukemia. Blood 86 (2): 819-24, 1995.
-
Giorgiani G, Bozzola M, Locatelli F, et al.: Role of busulfan and total body irradiation on growth of prepubertal children receiving bone marrow transplantation and results of treatment with recombinant human growth hormone. Blood 86 (2): 825-31, 1995.
-
Cohen A, Rovelli A, Van-Lint MT, et al.: Final height of patients who underwent bone marrow transplantation during childhood. Arch Dis Child 74 (5): 437-40, 1996.
-
Sanders JE, Guthrie KA, Hoffmeister PA, et al.: Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood 105 (3): 1348-54, 2005.
-
Cohen A, Rovelli A, Bakker B, et al.: Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood 93 (12): 4109-15, 1999.
-
Eggleston B, Patience M, Edwards S, et al.: Effect of myeloablative bone marrow transplantation on growth in children with sickle cell anaemia: results of the multicenter study of haematopoietic cell transplantation for sickle cell anaemia. Br J Haematol 136 (4): 673-6, 2007.
-
Reusch JE: Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. Am J Cardiol 90 (5A): 19G-26G, 2002.
-
Trevisan M, Liu J, Bahsas FB, et al.: Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol 148 (10): 958-66, 1998.
-
Lakka HM, Laaksonen DE, Lakka TA, et al.: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288 (21): 2709-16, 2002.
-
Taskinen M, Saarinen-Pihkala UM, Hovi L, et al.: Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet 356 (9234): 993-7, 2000.
-
Baker KS, Ness KK, Steinberger J, et al.: Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood 109 (4): 1765-72, 2007.
-
Eaton SB, Cordain L, Sparling PB: Evolution, body composition, insulin receptor competition, and insulin resistance. Prev Med 49 (4): 283-5, 2009.
-
Boirie Y: Physiopathological mechanism of sarcopenia. J Nutr Health Aging 13 (8): 717-23, 2009.
-
Baker KS, Chow E, Goodman P, et al.: Adverse Impact of hematopoietic cell transplantation (HCT) on body composition and insulin resistance (IR) is associated with increased cardiovascular risk. [Abstract] Biol Blood Marrow Transplant 17 (2 Suppl): A-61, S174, 2011.
-
Cook S, Weitzman M, Auinger P, et al.: Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med 157 (8): 821-7, 2003.
-
Genuth S, Alberti KG, Bennett P, et al.: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26 (11): 3160-7, 2003.
-
Grundy SM, Brewer HB Jr, Cleeman JI, et al.: Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109 (3): 433-8, 2004.
-
Petryk A, Bergemann TL, Polga KM, et al.: Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. J Clin Endocrinol Metab 91 (3): 899-905, 2006.
-
Bhatia S, Ramsay NK, Weisdorf D, et al.: Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant 22 (1): 87-90, 1998.
-
Nysom K, Holm K, Michaelsen KF, et al.: Bone mass after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant 25 (2): 191-6, 2000.
-
Daniels MW, Wilson DM, Paguntalan HG, et al.: Bone mineral density in pediatric transplant recipients. Transplantation 76 (4): 673-8, 2003.
-
Kaste SC, Shidler TJ, Tong X, et al.: Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant 33 (4): 435-41, 2004.
-
Perkins JL, Kunin-Batson AS, Youngren NM, et al.: Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer 49 (7): 958-63, 2007.
-
Carpenter PA, Hoffmeister P, Chesnut CH 3rd, et al.: Bisphosphonate therapy for reduced bone mineral density in children with chronic graft-versus-host disease. Biol Blood Marrow Transplant 13 (6): 683-90, 2007.
-
Weilbaecher KN: Mechanisms of osteoporosis after hematopoietic cell transplantation. Biol Blood Marrow Transplant 6 (2A): 165-74, 2000.
-
Rodgers C, Monroe R: Osteopenia and osteoporosis in pediatric patients after stem cell transplant. J Pediatr Oncol Nurs 24 (4): 184-9, 2007 Jul-Aug.
-
McClune BL, Polgreen LE, Burmeister LA, et al.: Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant 46 (1): 1-9, 2011.
-
Schulte CM, Beelen DW: Bone loss following hematopoietic stem cell transplantation: a long-term follow-up. Blood 103 (10): 3635-43, 2004.
-
Schimmer AD, Minden MD, Keating A: Osteoporosis after blood and marrow transplantation: clinical aspects. Biol Blood Marrow Transplant 6 (2A): 175-81, 2000.
-
Banfi A, Podestà M, Fazzuoli L, et al.: High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post-bone marrow transplantation osteopenia. Cancer 92 (9): 2419-28, 2001.
-
Castañeda S, Carmona L, Carvajal I, et al.: Reduction of bone mass in women after bone marrow transplantation. Calcif Tissue Int 60 (4): 343-7, 1997.
-
Lee WY, Baek KH, Rhee EJ, et al.: Impact of circulating bone-resorbing cytokines on the subsequent bone loss following bone marrow transplantation. Bone Marrow Transplant 34 (1): 89-94, 2004.
-
Baker KS, Gurney JG, Ness KK, et al.: Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood 104 (6): 1898-906, 2004.
-
Stern JM, Sullivan KM, Ott SM, et al.: Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant 7 (5): 257-64, 2001.
-
Ebeling PR, Thomas DM, Erbas B, et al.: Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res 14 (3): 342-50, 1999.
-
Tauchmanovà L, De Rosa G, Serio B, et al.: Avascular necrosis in long-term survivors after allogeneic or autologous stem cell transplantation: a single center experience and a review. Cancer 97 (10): 2453-61, 2003.
-
Kananen K, Volin L, Tähtelä R, et al.: Recovery of bone mass and normalization of bone turnover in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant 29 (1): 33-9, 2002.
-
Sharma S, Yang S, Rochester R, et al.: Prevalence of osteonecrosis and associated risk factors in children before allogeneic BMT. Bone Marrow Transplant 46 (6): 813-9, 2011.
-
Socié G, Cahn JY, Carmelo J, et al.: Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Société Française de Greffe de Moëlle (SFGM). Br J Haematol 97 (4): 865-70, 1997.
-
Bürger B, Beier R, Zimmermann M, et al.: Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)--experiences from trial ALL-BFM 95. Pediatr Blood Cancer 44 (3): 220-5, 2005.
-
Mattano LA Jr, Sather HN, Trigg ME, et al.: Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children's Cancer Group. J Clin Oncol 18 (18): 3262-72, 2000.
-
Li X, Brazauskas R, Wang Z, et al.: Avascular necrosis of bone after allogeneic hematopoietic cell transplantation in children and adolescents. Biol Blood Marrow Transplant 20 (4): 587-92, 2014.
-
Sanders J: Growth and development after hematopoietic cell transplantation . In: Appelbaum FR, Forman SJ, Negrin RS, et al., eds.: Thomas' Hematopoietic Cell Transplantation: Stem Cell Transplantation. 4th ed. Oxford, UK: Wiley-Blackwell, 2009, pp 1608-19.
-
Sanders JE, Flournoy N, Thomas ED, et al.: Marrow transplant experience in children with acute lymphoblastic leukemia: an analysis of factors associated with survival, relapse, and graft-versus-host disease. Med Pediatr Oncol 13 (4): 165-72, 1985.
-
Sanders JE, Hawley J, Levy W, et al.: Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 87 (7): 3045-52, 1996.
-
Carter A, Robison LL, Francisco L, et al.: Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant 37 (11): 1023-9, 2006.
-
Salooja N, Szydlo RM, Socie G, et al.: Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet 358 (9278): 271-6, 2001.
-
Loren AW, Chow E, Jacobsohn DA, et al.: Pregnancy after hematopoietic cell transplantation: a report from the late effects working committee of the Center for International Blood and Marrow Transplant Research (CIBMTR). Biol Blood Marrow Transplant 17 (2): 157-66, 2011.
-
Shalet SM, Didi M, Ogilvy-Stuart AL, et al.: Growth and endocrine function after bone marrow transplantation. Clin Endocrinol (Oxf) 42 (4): 333-9, 1995.
-
Schubert MA, Sullivan KM, Schubert MM, et al.: Gynecological abnormalities following allogeneic bone marrow transplantation. Bone Marrow Transplant 5 (6): 425-30, 1990.
-
Howell SJ, Shalet SM: Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr (34): 12-7, 2005.
-
Anserini P, Chiodi S, Spinelli S, et al.: Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant 30 (7): 447-51, 2002.
-
Panasiuk A, Nussey S, Veys P, et al.: Gonadal function and fertility after stem cell transplantation in childhood: comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. Br J Haematol 170 (5): 719-26, 2015.
-
Yoshihara S, Yanik G, Cooke KR, et al.: Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 13 (7): 749-59, 2007.
-
Chien JW, Duncan S, Williams KM, et al.: Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant 16 (1 Suppl): S106-14, 2010.
-
Hildebrandt GC, Fazekas T, Lawitschka A, et al.: Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant 46 (10): 1283-95, 2011.
-
Chien JW, Martin PJ, Gooley TA, et al.: Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 168 (2): 208-14, 2003.
-
Cerveri I, Zoia MC, Fulgoni P, et al.: Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 54 (2): 131-5, 1999.
-
Uhlving HH, Bang CL, Christensen IJ, et al.: Lung function after allogeneic hematopoietic stem cell transplantation in children: a longitudinal study in a population-based cohort. Biol Blood Marrow Transplant 19 (9): 1348-54, 2013.
-
Freudenberger TD, Madtes DK, Curtis JR, et al.: Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood 102 (10): 3822-8, 2003.
-
Chien JW, Zhao LP, Hansen JA, et al.: Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood 107 (5): 2200-7, 2006.
-
Hildebrandt GC, Granell M, Urbano-Ispizua A, et al.: Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 14 (1): 67-74, 2008.
-
Norman BC, Jacobsohn DA, Williams KM, et al.: Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant 46 (10): 1369-73, 2011.
-
Cooke KR, Yanik G: Lung injury following hematopoietic stem cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, et al., eds.: Thomas' Hematopoietic Cell Transplantation: Stem Cell Transplantation. 4th ed. Oxford, UK: Wiley-Blackwell, 2009, pp 1456-72.
-
Yanik GA, Mineishi S, Levine JE, et al.: Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant 18 (7): 1044-54, 2012.
-
Ellis MJ, Parikh CR, Inrig JK, et al.: Chronic kidney disease after hematopoietic cell transplantation: a systematic review. Am J Transplant 8 (11): 2378-90, 2008.
-
Choi M, Sun CL, Kurian S, et al.: Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer 113 (7): 1580-7, 2008.
-
Ando M, Ohashi K, Akiyama H, et al.: Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant 25 (1): 278-82, 2010.
-
Cohen EP, Piering WF, Kabler-Babbitt C, et al.: End-stage renal disease (ESRD)after bone marrow transplantation: poor survival compar ed to other causes of ESRD. Nephron 79 (4): 408-12, 1998.
-
Cohen EP, Irving AA, Drobyski WR, et al.: Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys 70 (5): 1546-51, 2008.
-
Wilson IB, Cleary PD: Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273 (1): 59-65, 1995.
-
Eisen M, Donald CA, Ware JE, et al.: Conceptualization and Measurement of Health for Children in the Health Insurance Study. Santa Monica, Calif: Rand Corporation, 1980.
-
Parsons SK, Barlow SE, Levy SL, et al.: Health-related quality of life in pediatric bone marrow transplant survivors: according to whom? Int J Cancer Suppl 12: 46-51, 1999.
-
Barrera M, Atenafu E, Hancock K: Longitudinal health-related quality of life outcomes and related factors after pediatric SCT. Bone Marrow Transplant 44 (4): 249-56, 2009.
-
Parsons SK, Shih MC, Duhamel KN, et al.: Maternal perspectives on children's health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol 31 (10): 1100-15, 2006 Nov-Dec.
-
Barrera M, Boyd-Pringle LA, Sumbler K, et al.: Quality of life and behavioral adjustment after pediatric bone marrow transplantation. Bone Marrow Transplant 26 (4): 427-35, 2000.
-
Jobe-Shields L, Alderfer MA, Barrera M, et al.: Parental depression and family environment predict distress in children before stem cell transplantation. J Dev Behav Pediatr 30 (2): 140-6, 2009.
-
Vrijmoet-Wiersma CM, Kolk AM, Grootenhuis MA, et al.: Child and parental adaptation to pediatric stem cell transplantation. Support Care Cancer 17 (6): 707-14, 2009.
-
Felder-Puig R, di Gallo A, Waldenmair M, et al.: Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: results of a longitudinal, multi-center study. Bone Marrow Transplant 38 (2): 119-26, 2006.
-
Parsons SK, Ratichek SJ, Rodday AM: Caring for the caregiver: eHealth interventions for parents of pediatric hematopoietic stem cell transplant recipients. [Abstract] Pediatr Blood Cancer 56 (7): 1157, 2011.
-
Brice L, Weiss R, Wei Y, et al.: Health-related quality of life (HRQoL): the impact of medical and demographic variables upon pediatric recipients of hematopoietic stem cell transplantation. Pediatr Blood Cancer 57 (7): 1179-85, 2011.
-
Kaplan SH, Barlow S, Spetter D: Assessing functional status and health-related quality of life among school-aged children: reliability and validity of a new self-reported measure. [Abstract] Qual Life Res 4 (5): 444-45, 1995.
-
Barrera M, Atenafu E, Doyle J, et al.: Differences in mothers' and fathers' health-related quality of life after pediatric SCT: a longitudinal study. Bone Marrow Transplant 47 (6): 855-9, 2012.
-
Feichtl RE, Rosenfeld B, Tallamy B, et al.: Concordance of quality of life assessments following pediatric hematopoietic stem cell transplantation. Psychooncology 19 (7): 710-7, 2010.
-
Löf CM, Winiarski J, Giesecke A, et al.: Health-related quality of life in adult survivors after paediatric allo-SCT. Bone Marrow Transplant 43 (6): 461-8, 2009.
-
Sanders JE, Hoffmeister PA, Storer BE, et al.: The quality of life of adult survivors of childhood hematopoietic cell transplant. Bone Marrow Transplant 45 (4): 746-54, 2010.
-
Duell T, van Lint MT, Ljungman P, et al.: Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Intern Med 126 (3): 184-92, 1997.
-
Schmidt GM, Niland JC, Forman SJ, et al.: Extended follow-up in 212 long-term allogeneic bone marrow transplant survivors. Issues of quality of life. Transplantation 55 (3): 551-7, 1993.
-
Helder DI, Bakker B, de Heer P, et al.: Quality of life in adults following bone marrow transplantation during childhood. Bone Marrow Transplant 33 (3): 329-36, 2004.
-
Michel G, Bordigoni P, Simeoni MC, et al.: Health status and quality of life in long-term survivors of childhood leukaemia: the impact of haematopoietic stem cell transplantation. Bone Marrow Transplant 40 (9): 897-904, 2007.
-
Ness KK, Bhatia S, Baker KS, et al.: Performance limitations and participation restrictions among childhood cancer survivors treated with hematopoietic stem cell transplantation: the bone marrow transplant survivor study. Arch Pediatr Adolesc Med 159 (8): 706-13, 2005.
-
Larsen RL, Barber G, Heise CT, et al.: Exercise assessment of cardiac function in children and young adults before and after bone marrow transplantation. Pediatrics 89 (4 Pt 2): 722-9, 1992.
-
Eames GM, Crosson J, Steinberger J, et al.: Cardiovascular function in children following bone marrow transplant: a cross-sectional study. Bone Marrow Transplant 19 (1): 61-6, 1997.
-
Hogarty AN, Leahey A, Zhao H, et al.: Longitudinal evaluation of cardiopulmonary performance during exercise after bone marrow transplantation in children. J Pediatr 136 (3): 311-7, 2000.
-
Fraser CJ, Bhatia S, Ness K, et al.: Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood 108 (8): 2867-73, 2006.
-
Rizzo JD, Wingard JR, Tichelli A, et al.: Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). Bone Marrow Transplant 37 (3): 249-61, 2006.
-
Bunin N, Small T, Szabolcs P, et al.: NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: persistent immune deficiency in pediatric transplant survivors. Biol Blood Marrow Transplant 18 (1): 6-15, 2012.
-
Dvorak CC, Gracia CR, Sanders JE, et al.: NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, & reproductive risks. Biol Blood Marrow Transplant 17 (12): 1725-38, 2011.
-
Parsons SK, Phipps S, Sung L, et al.: NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant 18 (2): 162-71, 2012.
Current Clinical TrialsCheck the list of NCI-supported cancer clinical trials that are now accepting patients with hematopoietic stem cell transplantation. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. Changes to This Summary (03 / 07 / 2017)The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Editorial changes were made to this summary. This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages. About This PDQ SummaryPurpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the use of hematopoietic cell transplantation in treating childhood cancer. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: - be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Childhood Hematopoietic Cell Transplantation are: - Thomas G. Gross, MD, PhD (National Cancer Institute)
- Michael A. Pulsipher, MD (Children's Hospital Los Angeles)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Hematopoietic Cell Transplantation. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/childhood-cancers/child-hct-hp-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389503] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2017-03-07 Last modified on: 8 September 2017
|
|