Childhood Central Nervous System Germ Cell Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]
Childhood Central Nervous System Germ Cell Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]Skip to the navigationGeneral Information About Childhood Central Nervous System (CNS) Germ Cell TumorsThe PDQ childhood brain tumor treatment summaries are organized primarily according to the World Health Organization classification of nervous system tumors.[1,2] For a full description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, refer to the PDQ summary on Childhood Brain and Spinal Cord Tumors Treatment Overview. Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[3] Childhood and adolescent cancer survivors require close follow-up because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.) Primary brain tumors are a diverse group of diseases that together constitute the most common solid tumor of childhood. Brain tumors are classified according to histology, but tumor location and extent of spread are important factors that affect treatment and prognosis. Immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Primary central nervous system (CNS) germ cell tumors (GCTs) are a heterogeneous group of neoplasms that account for 0.5% of all primary brain tumors, with approximately 90% of the cases occurring before age 20 years. They are broadly classified as germinomatous and nongerminomatous germ cell tumors (NGGCTs) based on clinicopathologic features. Alternatively, in Europe and Asia, these tumors are broadly classified into secreting and nonsecreting tumors, dependent on elevation of serum and cerebrospinal tumor markers.[1,2] Incidence In Western countries, GCTs represent less than 4% of primary brain tumors in children, while in series from Japan and Asia, CNS GCTs account for approximately 11% of pediatric CNS tumors.[4,5,6] Anatomy CNS GCTs usually arise in the pineal and/or suprasellar regions of the brain, as solitary or multiple lesions. Pineal region tumors occur twice as frequently as suprasellar tumors, but approximately 5% to 10% of patients have both suprasellar and pineal gland involvement at the time of diagnosis.[7] Involvement of both sites is most commonly seen in pure germinomas. Males have a higher incidence of GCT than do females, with males having a preponderance of pineal region primary tumors. Other areas that may be involved, though rare, include the basal ganglia, ventricles, thalamus, cerebral hemispheres, and the medulla.[8,9] 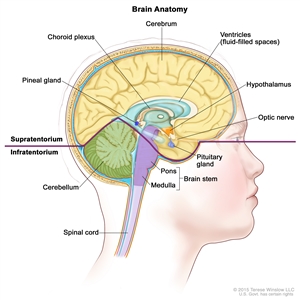
Figure 1. Anatomy of the inside of the brain. The supratentorium contains the cerebrum, ventricles (with cerebrospinal fluid shown in blue), choroid plexus, hypothalamus, pineal gland, pituitary gland, and optic nerve. The infratentorium contains the cerebellum and brain stem.
Tumor Biology In a study of 62 cases of intracranial GCT, next-generation sequencing, single nucleotide polymorphism array, and expression array have shown frequent mutations in the KIT/RAS signaling pathway (50% of cases) and the AKT/mTOR pathway (19% of cases).[10] Clinical Features Signs and symptoms of CNS GCTs depend on the location of the tumor in the brain, as follows: - Suprasellar region. Tumors arising in the suprasellar region often present with subtle or overt hormonal deficiencies and a protracted prodrome often lasting months to years. Diabetes insipidus caused by antidiuretic hormone deficiency occurs in 70% to 90% of patients and is the most common sentinel symptom; patients can usually compensate for this deficiency for months to years by drinking excessive amounts of fluid. Eventually, other hormonal symptoms and visual deficits emerge as the tumor expands dorsally and compresses or invades the optic chiasm.[6,11]
- Pineal region. Patients with tumors in the pineal region usually have a shorter history of symptoms than do patients with tumors of the suprasellar or basal ganglionic region, with weeks to months of symptoms that include raised intracranial pressure and diplopia related to tectal and aqueductal compression. Symptoms and signs unique to masses in the pineal and posterior third ventricular region include Parinaud syndrome (vertical gaze impairment, convergence nystagmus, and light-near pupillary response dissociation), headache, and nausea and vomiting.
- Multifocal or bifocal tumors. Patients with multifocal or bifocal primaries may present with both suprasellar and pineal region syndromes.[6]
Nonspecific symptoms such as enuresis, anorexia, and psychiatric complaints can lead to delays in diagnosis, whereas signs of increased intracranial pressure or visual changes tend to result in earlier diagnosis.[12] Diagnostic Evaluation Radiographic characteristics of CNS GCTs cannot reliably differentiate germinomas from NGGCTs or other CNS tumors. The diagnosis of GCTs is based on the following: - Clinical signs and symptoms.
- Tumor markers.
- Neuroimaging.
- Cytological cerebrospinal fluid (CSF) and histological confirmation.
Patients with a suspected CNS GCT are diagnosed using the following tests: - Magnetic resonance imaging (MRI) of brain and spine with gadolinium.
- Alpha-fetoprotein (AFP) and beta subunit human chorionic gonadotropin (beta-HCG) in both serum and CSF. If preoperative lumbar CSF can be obtained safely and tumor markers are elevated, this may obviate the need for upfront surgery. Lumbar CSF is preferred and is more sensitive than serum markers for beta-HCG.[13] When the patient presents with hydrocephalus requiring CSF diversion, CSF tumor markers can be obtained by ventricular CSF sampling at the time of surgery. (Refer to the Cellular Classification of Childhood CNS Germ Cell Tumors section of this summary for more information.)
- Lumbar CSF cytology.
- Evaluation of pituitary/hypothalamic function.
- Visual-field examinations for suprasellar or hypothalamic tumors.
A baseline neuropsychologic examination is also performed when symptoms of endocrine deficiency and raised intracranial pressure are resolved. Diagnosis of GCTs often requires a tumor biopsy, except in cases with characteristic increased tumor markers in the serum and/or CSF. When the tumor markers are negative or mildly elevated but below diagnostic criteria, or if there is any noncharacteristic finding, a tumor biopsy is performed. It is crucial that appropriate staging is determined and that pure germinomas are distinguished from NGGCTs. The chemotherapy and radiation treatment plan differs significantly depending on GCT category and extent of disease. References:
-
Louis DN, Ohgaki H, Wiestler OD, et al., eds.: WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: IARC Press, 2007.
-
Louis DN, Ohgaki H, Wiestler OD, et al.: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114 (2): 97-109, 2007.
-
Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
-
Matsutani M, Sano K, Takakura K, et al.: Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86 (3): 446-55, 1997.
-
Matsutani M; Japanese Pediatric Brain Tumor Study Group: Combined chemotherapy and radiation therapy for CNS germ cell tumors--the Japanese experience. J Neurooncol 54 (3): 311-6, 2001.
-
Hoffman HJ, Otsubo H, Hendrick EB, et al.: Intracranial germ-cell tumors in children. J Neurosurg 74 (4): 545-51, 1991.
-
Weksberg DC, Shibamoto Y, Paulino AC: Bifocal intracranial germinoma: a retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys 82 (4): 1341-51, 2012.
-
Goodwin TL, Sainani K, Fisher PG: Incidence patterns of central nervous system germ cell tumors: a SEER Study. J Pediatr Hematol Oncol 31 (8): 541-4, 2009.
-
Villano JL, Propp JM, Porter KR, et al.: Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro Oncol 10 (2): 121-30, 2008.
-
Wang L, Yamaguchi S, Burstein MD, et al.: Novel somatic and germline mutations in intracranial germ cell tumours. Nature 511 (7508): 241-5, 2014.
-
Afzal S, Wherrett D, Bartels U, et al.: Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol 97 (3): 393-9, 2010.
-
Crawford JR, Santi MR, Vezina G, et al.: CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology 68 (20): 1668-73, 2007.
-
Allen J, Chacko J, Donahue B, et al.: Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer 59 (7): 1180-2, 2012.
Cellular Classification of Childhood CNS Germ Cell TumorsThe pathogenesis of intracranial germ cell tumors (GCTs) is unknown. The germ cell theory proposes that central nervous system (CNS) GCTs arise from primordial germ cells that have aberrantly migrated and undergone malignant transformation. An alternative hypothesis, the embryonic cell theory, proposes that GCTs arise from a pluripotent embryonic cell that escapes normal developmental signals and progresses to CNS GCTs.[1,2] Recent investigations comparing the genomic alterations in GCTs found similar copy number alterations whether the GCT was systemic or CNS based.[3] The World Health Organization has classified CNS GCTs into germinomas and the following nongerminomatous germ cell tumor (NGGCT) groups:[4] - Choriocarcinoma.
- Embryonal carcinoma.
- Mixed germ cell tumor.
- Teratoma.
- Immature.
- Mature.
- Teratoma with malignant transformation.
- Yolk sac tumor.
In addition to the microscopic appearance of the various CNS GCTs, tumor markers (proteins, such as alpha-fetoprotein [AFP] and beta subunit human chorionic gonadotropin [beta-HCG], secreted by the tumor cells) found in the serum and cerebrospinal fluid (CSF) aid in diagnosis (refer to Tables 1 and 2). The diagnosis and classification of CNS GCTs can be made on the basis of histology alone, tumor markers alone, or a combination of both.[3,5,6,7] Favorable-risk germinomas can secrete low levels of beta-HCG. NGGCTs frequently contain germinomatous plus other malignant GCT components, such as embryonal carcinoma, yolk sac or endodermal sinus tumor, and choriocarcinoma. Table 1. Immunohistochemical Markers| Tumor Type | Beta-HCG | AFP | PLAP | c-kit |
|---|
| AFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; PLAP = placental alkaline phosphatase; + = positive; - = negative; ± = equivocal. | | Choriocarcinoma | + | - | ± | - | | Embryonal carcinoma | - | - | + | - | | Germinoma (syncytiotrophoblastic) | + | - | ± | + | | Immature teratoma | ± | ± | - | ± | | Mature teratoma | - | - | - | - | | Mixed germ cell tumor | ± | ± | ± | ± | | Pure germinoma | - | - | ± | + | | Yolk sac tumor | - | + | ± | - | Table 2. Serum and Cerebrospinal Fluid Markers| Tumor Type | Beta-HCG | AFP |
|---|
| AFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; + = positive; - = negative; ± = equivocal; +++ = strongly positive; (±) equivocal, not diagnostic; (+) = positive, not diagnostic. | | Choriocarcinoma | +++ | - | | Embryonal carcinoma | + | + | | Germinoma | (±) | - | | Teratoma | - | (+) | | Yolk sac tumor | - | +++ | Elevations of tumor markers along with imaging findings are used as surrogate diagnostic markers for CNS GCT and may obviate the need for histologic diagnosis. The tumor markers AFP and beta-HCG are the most useful, although other markers, such as placental alkaline phosphatase and c-kit, are being investigated. Distinguishing between different GCT types by CSF protein marker levels alone is somewhat arbitrary, and standards vary. For example, groups in the United States and Europe consider tumors as secreting if serum and/or CSF AFP levels are 10 ng/dL or higher and/or serum and/or CSF beta-HCG levels are 50 mIU/mL or higher, whereas several European and Asian groups designate tumors with serum and/or CSF AFP levels of 50 ng/mL or higher and/or beta-HCG levels of 100 mIU/mL or higher as secreting GCTs. Pure germinomas and teratomas usually present with negative markers, but very low levels of beta-HCG can be detected in germinomas.[8] The use of tumor markers and histology in GCT clinical trials is evolving. For example, in the COG-ACNS1123 [NCT01602666] trial, patients are eligible for assignment to the germinoma regimen without biopsy confirmation if they have one of the following: - Either pineal region tumors or suprasellar primary tumors, normal AFP levels, and beta-HCG levels between 5 and 50 mIU/mL in serum and/or CSF.
- Bifocal presentation (pineal and suprasellar), diabetes insipidus, normal AFP levels, and beta-HCG levels lower than 100 mIU/mL in CSF.
Alternative classification schemes for CNS GCTs have been proposed by others, including the Japanese Pediatric Brain Tumor Study Group for CNS GCTs, who based their stratification on the prognostic grouping of the differing histologic variants as shown in Table 3. Pure germinomas and mature teratomas fall into the good prognostic group; choriocarcinoma, yolk sac tumor, embryonal carcinoma, or mixtures of these three histologic subtypes fall into the poor prognostic group.[6] Table 3. Japanese Pediatric Brain Tumor Study Group Classification| Prognostic Group | Tumor Type |
|---|
| Good | Germinoma, pure | | Mature teratoma | | Intermediate | Germinoma with syncytiotrophoblastic giant cells | | Immature teratoma | | Mixed tumors mainly composed of germinoma or teratoma | | Teratoma with malignant transformation | | Poor | Choriocarcinoma | | Embryonal carcinoma | | Mixed tumors composed of choriocarcinoma, yolk sac tumor, or embryonal carcinoma | | Yolk sac tumor | References:
-
Sano K, Matsutani M, Seto T: So-called intracranial germ cell tumours: personal experiences and a theory of their pathogenesis. Neurol Res 11 (2): 118-26, 1989.
-
Teilum G: Embryology of ovary, testis, and genital ducts. In: Teilum G: Special Tumors of Ovary and Testis and Related Extragonadal Lesions: Comparative Pathology and Histological Identification. Philadelphia, Pa: J. B. Lippincott, 1976, pp 15-30.
-
Schneider DT, Zahn S, Sievers S, et al.: Molecular genetic analysis of central nervous system germ cell tumors with comparative genomic hybridization. Mod Pathol 19 (6): 864-73, 2006.
-
Miyanohara O, Takeshima H, Kaji M, et al.: Diagnostic significance of soluble c-kit in the cerebrospinal fluid of patients with germ cell tumors. J Neurosurg 97 (1): 177-83, 2002.
-
Matsutani M, Sano K, Takakura K, et al.: Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86 (3): 446-55, 1997.
-
Rosenblum MK, Matsutani M, Van Meir EG: CNS germ cell tumours. In: Kleihues P, Cavenee WK, eds.: Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer, 2000, pp 208-14.
-
Allen J, Chacko J, Donahue B, et al.: Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer 59 (7): 1180-2, 2012.
-
Frazier AL, Olson TA, Schneider DT, et al.: Germ cell tumors. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 899-918.
Stage Information for Childhood CNS Germ Cell TumorsThere is no universally accepted staging system for germ cell tumors (GCTs), but a modified Chang Staging System has been traditionally used.[1] Staging evaluation of central nervous system GCTs includes the following: Patients with localized disease and negative CSF cytology are considered to be M0 (metastatic-negative); patients with positive CSF cytology or patients with drop metastasis (spinal or cranial subarachnoid metastases that arise from intracranial lesions) are considered to be M+ (metastatic-positive). Appropriate staging is crucial because patients with metastatic disease may receive higher total doses of radiation and more extended radiation fields. GCTs may be disseminated throughout the neuraxis at the time of diagnosis or at any disease stage. Several unusual patterns of spread may occur in germinomas, such as subependymal dissemination in the lateral or third ventricles and parenchymal infiltration. Rarely, extracranial spread to lung and bone has also been reported.[3,4] Staging of patients with bifocal intracranial germinomas limited to the suprasellar and pineal region remains controversial, with some classifying these tumors as localized disease and others classifying such presentations as disseminated disease.[5] References:
-
Calaminus G, Kortmann R, Worch J, et al.: SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15 (6): 788-96, 2013.
-
Fujimaki T, Mishima K, Asai A, et al.: Levels of beta-human chorionic gonadotropin in cerebrospinal fluid of patients with malignant germ cell tumor can be used to detect early recurrence and monitor the response to treatment. Jpn J Clin Oncol 30 (7): 291-4, 2000.
-
Jennings MT, Gelman R, Hochberg F: Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63 (2): 155-67, 1985.
-
Gay JC, Janco RL, Lukens JN: Systemic metastases in primary intracranial germinoma. Case report and literature review. Cancer 55 (11): 2688-90, 1985.
-
Weksberg DC, Shibamoto Y, Paulino AC: Bifocal intracranial germinoma: a retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys 82 (4): 1341-51, 2012.
Treatment Option Overview for Childhood CNS Germ Cell TumorsTeratomas, germinomas, and other nongerminomatous germ cell tumors (NGGCTs) have differing prognoses and require different treatment regimens. Studies have observed the following:[1,2,3,4,5] - For children older than 3 years and adults, radiation therapy has been an important component of therapy for germinomas and NGGCTs, although the total dose and fields are debated.
- Germinomas are curable with craniospinal irradiation and local site-boost radiation therapy; however, there is a trend in clinical trials to use neoadjuvant or preirradiation chemotherapy to allow reduced doses and volumes of radiation therapy in patients whose tumors have a complete response to chemotherapy to reduce long-term radiation therapy-related effects. Patients with localized germinomas are effectively treated with whole-ventricular irradiation supplemented with tumor site-boost radiation therapy; focal irradiation to the tumor bed, regardless of response to chemotherapy, is considered inadequate treatment.[6]
- For NGGCTs, the combined use of more intensive neoadjuvant chemotherapy followed by craniospinal radiation in clinical trials has resulted in excellent survival rates in the last decade.
- Germ cell tumors (GCTs) arising in the central nervous system, similar to gonadal and extragonadal GCTs, have demonstrated sensitivity to chemotherapy.
Table 4. Treatment Options for Childhood Central Nervous System (CNS) Germ Cell Tumors (GCTs)| Treatment Group | Treatment Options |
|---|
| Newly diagnosed childhood germinomas | Radiation therapy | | Neoadjuvant chemotherapy followed by response-based radiation therapy | | Newly diagnosed childhood teratomas | Surgery | | Adjuvant therapy, for patients who had a subtotal resection (controversial): | | -Focal radiation therapy | | -Chemotherapy | | -Stereotactic radiosurgery | | Newly diagnosed childhood nongerminomatous GCTs | Chemotherapy followed by radiation therapy | | Surgery | | Recurrent childhood CNS GCTs | Chemotherapy followed by radiation therapy | | High-dose chemotherapy with stem cell rescue | References:
-
Osuka S, Tsuboi K, Takano S, et al.: Long-term outcome of patients with intracranial germinoma. J Neurooncol 83 (1): 71-9, 2007.
-
Allen JC, Kim JH, Packer RJ: Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg 67 (1): 65-70, 1987.
-
Kellie SJ, Boyce H, Dunkel IJ, et al.: Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol 22 (5): 846-53, 2004.
-
Calaminus G, Kortmann R, Worch J, et al.: SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15 (6): 788-96, 2013.
-
Calaminus G, Bamberg M, Harms D, et al.: AFP/beta-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics 36 (2): 71-7, 2005.
-
Joo JH, Park JH, Ra YS, et al.: Treatment outcome of radiation therapy for intracranial germinoma: adaptive radiation field in relation to response to chemotherapy. Anticancer Res 34 (10): 5715-21, 2014.
Treatment of Newly Diagnosed Childhood CNS GerminomasTreatment Options for Newly Diagnosed Childhood CNS Germinomas Treatment options for newly diagnosed childhood CNS germinomas include the following: - Radiation therapy.
- Neoadjuvant chemotherapy followed by response-based radiation therapy.
Radiation therapy Germinomas are highly radiosensitive and have been traditionally treated with radiation therapy alone; historically, craniospinal irradiation with a boost to the region of the primary tumor has been utilized. This has resulted in 5-year overall survival rates of greater than 90%.[1]; [2][Level of evidence: 2A]; [3,4][Level of evidence: 3iA] These excellent survival rates have allowed investigators to focus on reducing radiation treatment volume and intensity in an attempt to decrease late effects.[2,5,6] Patterns of relapse after craniospinal irradiation versus reduced-volume radiation therapy (whole-brain or whole-ventricular radiation therapy) have strongly suggested that craniospinal irradiation is not necessary for localized germinomas.[7,8] On the basis of these results, the treatment for patients with localized pure germinomas has been modified to cover the whole ventricular system (24 Gy) followed by a boost to the primary site, rather than to deliver radiation therapy to the entire craniospinal axis or even to the whole brain. This change has not resulted in worse outcomes and is expected to minimize the acute and long-term toxicity of radiation therapy. Focal radiation therapy after neoadjuvant chemotherapy results in inferior outcomes compared with whole-brain or whole-ventricle radiation therapy; therefore, focal radiation therapy is not recommended.[6] Neoadjuvant chemotherapy followed by response-based radiation therapy Chemotherapy has been explored in an effort to reduce radiation therapy doses and associated neurodevelopmental morbidity. Several studies have confirmed the feasibility of this approach for maintaining excellent survival rates, but the number of treated patients is still small.[9,10,11]; [12][Level of evidence: 2A]; [13][Level of evidence: 3iiiC] Chemotherapy agents such as cyclophosphamide, ifosfamide, etoposide, cisplatin, and carboplatin are highly active in central nervous system (CNS) germinomas. Patients receiving chemotherapy agents that require hyperhydration (e.g., cyclophosphamide, ifosfamide, and cisplatin) are often quite challenging to manage because of the high prevalence of diabetes insipidus in this population.[14] An international group of investigators have explored a chemotherapy-only approach primarily for younger children. They were able to achieve a complete response in 84% of patients with germinomas treated with chemotherapy alone. Fifty percent of these patients relapsed or progressed; many recurrences were local, local plus ventricular, and ventricular alone and/or with leptomeningeal dissemination throughout the CNS, which required further therapy, including radiation.[15] Subsequent studies have continued to support the need for radiation therapy after chemotherapy and the likely requirement for whole-ventricular irradiation (24 Gy) with local tumor site boost (total dose of 40 Gy).[16][Level of evidence: 2A]; [17][Level of evidence: 3iiiA] Excellent results have also been reported for patients with metastatic germinomas who received chemotherapy followed by 24 Gy of craniospinal irradiation.[18][Level of evidence: 2A] Optimal management of bifocal lesions is unclear. A meta-analysis of 60 patients demonstrated excellent progression-free survival after craniospinal irradiation alone. Chemotherapy plus localized radiation therapy, including whole-ventricular irradiation, also resulted in excellent disease control.[19][Level of evidence: 3iiDiii] Treatment Options Under Clinical Evaluation for Newly Diagnosed Childhood CNS Germinomas The following is an example of a national and/or institutional clinical trial that is currently being conducted or is under analysis. Information about ongoing clinical trials is available from the NCI website. Treatment options under clinical evaluation for newly diagnosed childhood CNS germinomas include the following: - COG-ACNS1123 (NCT01602666) (Chemotherapy Followed by Radiation Therapy in Treating Younger Patients With Newly Diagnosed Localized CNS Germ Cell Tumors [GCTs]): COG-ACNS1123 is a Children's Oncology Group cooperative multi-institutional trial. This phase II trial of response-based radiation therapy for patients with localized CNS GCTs will compare the event-free survival and overall survival rates of a short course of chemotherapy followed by response-based, whole-ventricular radiation therapy, with a boost to the primary site. For patients who obtain a complete response after chemotherapy, the whole-ventricular radiation dose will be 25% lower than the standard whole-ventricular dose; for patients who have less than a complete response after chemotherapy, the standard whole-ventricular dose will be used, with or without second-look surgery.
References:
-
Shibamoto Y, Abe M, Yamashita J, et al.: Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys 15 (2): 285-90, 1988.
-
Cho J, Choi JU, Kim DS, et al.: Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol 91 (1): 75-9, 2009.
-
Huang PI, Chen YW, Wong TT, et al.: Extended focal radiotherapy of 30 Gy alone for intracranial synchronous bifocal germinoma: a single institute experience. Childs Nerv Syst 24 (11): 1315-21, 2008.
-
Eom KY, Kim IH, Park CI, et al.: Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas: modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys 71 (3): 667-71, 2008.
-
Chen MJ, Santos Ada S, Sakuraba RK, et al.: Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular irradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. Int J Radiat Oncol Biol Phys 76 (2): 608-14, 2010.
-
Joo JH, Park JH, Ra YS, et al.: Treatment outcome of radiation therapy for intracranial germinoma: adaptive radiation field in relation to response to chemotherapy. Anticancer Res 34 (10): 5715-21, 2014.
-
Rogers SJ, Mosleh-Shirazi MA, Saran FH: Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol 6 (7): 509-19, 2005.
-
Shikama N, Ogawa K, Tanaka S, et al.: Lack of benefit of spinal irradiation in the primary treatment of intracranial germinoma: a multiinstitutional, retrospective review of 180 patients. Cancer 104 (1): 126-34, 2005.
-
Kretschmar C, Kleinberg L, Greenberg M, et al.: Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer 48 (3): 285-91, 2007.
-
Allen JC, DaRosso RC, Donahue B, et al.: A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer 74 (3): 940-4, 1994.
-
Buckner JC, Peethambaram PP, Smithson WA, et al.: Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 17 (3): 933-40, 1999.
-
Khatua S, Dhall G, O'Neil S, et al.: Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer 55 (1): 42-6, 2010.
-
O'Neil S, Ji L, Buranahirun C, et al.: Neurocognitive outcomes in pediatric and adolescent patients with central nervous system germinoma treated with a strategy of chemotherapy followed by reduced-dose and volume irradiation. Pediatr Blood Cancer 57 (4): 669-73, 2011.
-
Afzal S, Wherrett D, Bartels U, et al.: Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol 97 (3): 393-9, 2010.
-
Balmaceda C, Heller G, Rosenblum M, et al.: Chemotherapy without irradiation--a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 14 (11): 2908-15, 1996.
-
da Silva NS, Cappellano AM, Diez B, et al.: Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer 54 (3): 377-83, 2010.
-
Alapetite C, Brisse H, Patte C, et al.: Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol 12 (12): 1318-25, 2010.
-
Calaminus G, Kortmann R, Worch J, et al.: SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15 (6): 788-96, 2013.
-
Weksberg DC, Shibamoto Y, Paulino AC: Bifocal intracranial germinoma: a retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys 82 (4): 1341-51, 2012.
Treatment of Newly Diagnosed Childhood CNS TeratomasTreatment Options for Newly Diagnosed Childhood CNS Teratomas Teratomas are designated as mature or immature based on the absence or presence of differentiated tissues. The Japanese Pediatric Brain Tumor Study Group stratifies teratomas for classification and intensity of treatment (chemotherapy and radiation) into the good-risk (mature teratomas) and intermediate-risk (immature teratomas) groups (refer to Table 3), while the Children's Oncology Group includes immature teratomas with other nongerminomatous germ cell tumors. Treatment options for newly diagnosed childhood CNS teratomas include the following: - Surgery.
- Adjuvant therapy, for patients who had a subtotal resection (controversial).
- Focal radiation therapy.
- Chemotherapy.
- Stereotactic radiosurgery.
The primary treatment for teratomas is maximal surgical resection. Adjuvant treatment in the form of focal radiation therapy and/or adjuvant chemotherapy for subtotally resected tumors is controversial, with small institutional series suggesting potential utility for the use of stereotactic radiosurgery.[1,2][Level of evidence: 3iA] References:
-
Huang X, Zhang R, Zhou LF: Diagnosis and treatment of intracranial immature teratoma. Pediatr Neurosurg 45 (5): 354-60, 2009.
-
Lee YH, Park EK, Park YS, et al.: Treatment and outcomes of primary intracranial teratoma. Childs Nerv Syst 25 (12): 1581-7, 2009.
Treatment of Newly Diagnosed Childhood CNS Nongerminomatous Germ Cell TumorsThe prognosis for children with CNS nongerminomatous germ cell tumors (NGGCTs) remains inferior to germinomas, but the differential is diminishing with the recent addition of multimodality therapy. With the current treatment regimens, the 10-year overall survival (OS) for NGGCTs is between 70% and 80%.[1,2] NGGCTs are radiosensitive, but survival after standard craniospinal irradiation alone has been poor, ranging from 20% to 45% at 5 years. Of the patients with NGGCTs who relapse, most relapse within 18 months. Treatment Options for Newly Diagnosed Childhood CNS NGGCTs Treatment options for newly diagnosed childhood CNS NGGCTs include the following: - Chemotherapy followed by radiation therapy.
- Surgery, for tumors that do not respond to treatment or for tumors that increase in size after therapy (possible growing teratoma syndrome).
The optimal treatment regimen for CNS NGGCTs remains unclear. Chemotherapy followed by radiation therapy Anticancer agents that have been used include carboplatin, etoposide, bleomycin, ifosfamide, and vinblastine in different combinations. The use of chemotherapy before radiation therapy has increased survival rates, but the specific chemotherapy regimen and length of therapy and the optimal radiation field, timing, and dose remain under investigation.[1,3,4] Some investigators have proposed radiation therapy fields that are smaller than craniospinal irradiation (e.g., whole ventricular with boost radiation therapy to the local tumor site) for nondisseminated NGGCT patients. Controversy exists over the pattern of relapse for patients treated with chemotherapy and focal radiation.[1,2,5,6] A COG study (ACNS0122 [NCT00047320]) evaluated neoadjuvant chemotherapy followed by radiation therapy for children with localized NGGCTs.[7] Neoadjuvant chemotherapy consisted of six courses using carboplatin/etoposide alternating with ifosfamide/etoposide. After chemotherapy was completed, responding patients received 36 Gy of craniospinal radiation therapy, with 54 Gy to the tumor bed. On the basis of central review, 87% of patients showed either partial response (PR) or complete response (CR). For the 102 eligible patients in the study, 5-year event-free survival (EFS) was 84% ± 4%, and OS was 93% ± 3%. At 3 years, the EFS was 92% and the OS was 98% for all patients who achieved CR or PR either after induction chemotherapy or with absence of malignant elements documented during second-look surgery. Surgery Commonly, patients treated with chemotherapy may have normalization of tumor markers with a less-than-complete radiographic response. A second-look surgery can help determine if the residual mass contains teratoma, fibrosis, or residual NGGCT.[2,8] If second-look surgery finds mature teratoma or fibrosis after chemotherapy, the general approach is to proceed with radiation therapy as if the patient had achieved a CR to chemotherapy. However, if active tumor is observed, then alternative treatment approaches are generally considered.[7] Occasionally, a mass continues to expand in size even though tumor markers may have normalized; this expansion may be caused by the growing teratoma syndrome and not a failure to treat the NGGCT component.[7,9,10] In such circumstances, surgery is usually required for debulking and histologic confirmation. Treatment Options Under Clinical Evaluation for Newly Diagnosed Childhood CNS NGGCTs The following is an example of a national and/or institutional clinical trial that is currently being conducted or is under analysis. Information about ongoing clinical trials is available from the NCI website. Treatment options under clinical evaluation for newly diagnosed childhood CNS NGGCTs include the following: - COG-ACNS1123 (NCT01602666) (Chemotherapy Followed by Radiation Therapy in Treating Younger Patients With Newly Diagnosed Localized Central Nervous System [CNS] GCTs): COG-ACNS1123 is a Children's Oncology Group cooperative multi-institutional trial. This phase II trial of response-based radiation therapy for patients with localized CNS GCTs will compare the event-free survival and OS rates of a short course of chemotherapy followed by response-based, whole-ventricular radiation, with a boost to the primary site. For patients who obtain a complete response after chemotherapy, the whole-ventricular radiation dose will be 25% lower than the standard whole-ventricular dose; for patients who have less than a complete response after chemotherapy, the standard whole-ventricular dose will be used, with or without second-look surgery.
References:
-
Robertson PL, DaRosso RC, Allen JC: Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol 32 (1): 71-80, 1997.
-
Baranzelli M, Patte C, Bouffet E, et al.: Carboplatin-based chemotherapy (CT) and focal irradiation (RT) in primary germ cell tumors (GCT): A French Society of Pediatric Oncology (SFOP) experience (meeting abstract). [Abstract] Proceedings of the American Society of Clinical Oncology 18: A-538, 140A, 1999.
-
Matsutani M; Japanese Pediatric Brain Tumor Study Group: Combined chemotherapy and radiation therapy for CNS germ cell tumors--the Japanese experience. J Neurooncol 54 (3): 311-6, 2001.
-
Calaminus G, Bamberg M, Jürgens H, et al.: Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non-germinomatous germ cell tumors: results of the German Cooperative Trial MAKEI 89. Klin Padiatr 216 (3): 141-9, 2004 May-Jun.
-
Aoyama H, Shirato H, Ikeda J, et al.: Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol 20 (3): 857-65, 2002.
-
Kim JW, Kim WC, Cho JH, et al.: A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys 84 (3): 625-31, 2012.
-
Goldman S, Bouffet E, Fisher PG, et al.: Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study. J Clin Oncol 33 (22): 2464-71, 2015.
-
Oya S, Saito A, Okano A, et al.: The pathogenesis of intracranial growing teratoma syndrome: proliferation of tumor cells or formation of multiple expanding cysts? Two case reports and review of the literature. Childs Nerv Syst 30 (8): 1455-61, 2014.
-
Yagi K, Kageji T, Nagahiro S, et al.: Growing teratoma syndrome in a patient with a non-germinomatous germ cell tumor in the neurohypophysis--case report. Neurol Med Chir (Tokyo) 44 (1): 33-7, 2004.
-
Kim CY, Choi JW, Lee JY, et al.: Intracranial growing teratoma syndrome: clinical characteristics and treatment strategy. J Neurooncol 101 (1): 109-15, 2011.
Treatment of Recurrent Childhood CNS Germ Cell TumorsThe most common type of relapse is local recurrence at the primary tumor site, but 30% of relapses are outside the primary site and/or combined with leptomeningeal spread. The outcome for patients with relapse, especially those with nongerminomatous germ cell tumors (NGGCTs), remains poor. Treatment Options for Recurrent Childhood Central Nervous System (CNS) Germ Cell Tumors (GCTs) Treatment options for recurrent childhood CNS GCTs include the following: - Chemotherapy followed by radiation therapy.
- High-dose chemotherapy with stem cell rescue.
Patients with germinomas that were treated initially with chemotherapy only can benefit from chemotherapy followed by radiation therapy.[1,2] Re-irradiation after chemotherapy at recurrence has been utilized.[2,3] For pure germinoma patients who previously received radiation therapy, myeloablative chemotherapy with stem cell rescue has been used.[4,5] High-dose chemotherapy and autologous stem cell rescue may also have curative potential for some patients with relapsed systemic NGGCTs.[4,5,6,7] Enrollment on clinical trials should be considered for all patients with recurrent disease. Information about ongoing clinical trials is available from the NCI website. References:
-
Merchant TE, Sherwood SH, Mulhern RK, et al.: CNS germinoma: disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys 46 (5): 1171-6, 2000.
-
Sawamura Y, Ikeda JL, Tada M, et al.: Salvage therapy for recurrent germinomas in the central nervous system. Br J Neurosurg 13 (4): 376-81, 1999.
-
Hu YW, Huang PI, Wong TT, et al.: Salvage treatment for recurrent intracranial germinoma after reduced-volume radiotherapy: a single-institution experience and review of the literature. Int J Radiat Oncol Biol Phys 84 (3): 639-47, 2012.
-
Siegert W, Beyer J, Strohscheer I, et al.: High-dose treatment with carboplatin, etoposide, and ifosfamide followed by autologous stem-cell transplantation in relapsed or refractory germ cell cancer: a phase I/II study. The German Testicular Cancer Cooperative Study Group. J Clin Oncol 12 (6): 1223-31, 1994.
-
Modak S, Gardner S, Dunkel IJ, et al.: Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol 22 (10): 1934-43, 2004.
-
Beyer J, Kramar A, Mandanas R, et al.: High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol 14 (10): 2638-45, 1996.
-
Motzer RJ, Mazumdar M, Bosl GJ, et al.: High-dose carboplatin, etoposide, and cyclophosphamide for patients with refractory germ cell tumors: treatment results and prognostic factors for survival and toxicity. J Clin Oncol 14 (4): 1098-105, 1996.
Long-Term Effects of Childhood CNS Germ Cell TumorsA significant proportion of children with central nervous system (CNS) germ cell tumors (GCTs) present with endocrinopathies, including diabetes insipidus and panhypopituitarism. In most cases, these endocrinopathies are permanent despite tumor control and will need continuous hormone replacement therapy.[1,2,3] Although significant improvements in the overall survival of patients with CNS GCTs have occurred, patients face significant late effects based on the location of the primary tumor and its treatment. Treatment-related late effects include the following: - Each chemotherapeutic agent has its own characteristic long-term side effects.
- Radiation therapy to the areas commonly affected by GCTs is known to cause a decline in patient performance status, visual-field impairments, extraocular movement disturbances, endocrine disorders, learning disabilities, and stroke.[4,5,6,7,8]
- Second tumors have been identified in this population, some of which are thought to be related to previous irradiation.[8,9]
Current clinical trials and therapeutic approaches are directed at minimizing the long-term sequelae of the treatment of CNS GCTs. Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors. References:
-
Rosenblum MK, Matsutani M, Van Meir EG: CNS germ cell tumours. In: Kleihues P, Cavenee WK, eds.: Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer, 2000, pp 208-14.
-
Hoffman HJ, Otsubo H, Hendrick EB, et al.: Intracranial germ-cell tumors in children. J Neurosurg 74 (4): 545-51, 1991.
-
Jennings MT, Gelman R, Hochberg F: Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63 (2): 155-67, 1985.
-
Osuka S, Tsuboi K, Takano S, et al.: Long-term outcome of patients with intracranial germinoma. J Neurooncol 83 (1): 71-9, 2007.
-
Balmaceda C, Finlay J: Current advances in the diagnosis and management of intracranial germ cell tumors. Curr Neurol Neurosci Rep 4 (3): 253-62, 2004.
-
Odagiri K, Omura M, Hata M, et al.: Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys 84 (3): 632-8, 2012.
-
Liang SY, Yang TF, Chen YW, et al.: Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol 15 (11): 1543-51, 2013.
-
Acharya S, DeWees T, Shinohara ET, et al.: Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol 17 (5): 741-6, 2015.
-
Jabbour SK, Zhang Z, Arnold D, et al.: Risk of second tumor in intracranial germinoma patients treated with radiation therapy: the Johns Hopkins experience. J Neurooncol 91 (2): 227-32, 2009.
Current Clinical TrialsCheck the list of NCI-supported cancer clinical trials that are now accepting patients with childhood central nervous system germ cell tumor. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria. General information about clinical trials is also available from the NCI website. Changes to This Summary (09 / 23 / 2016)The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Long-Term Effects of Childhood CNS Germ Cell Tumors (GCTs) Revised text to state that radiation therapy to the areas commonly affected by GCTs is known to cause a decline in patient performance status, visual-field impairments, extraocular movement disturbances, endocrine disorders, learning disabilities, and stroke (cited Acharya et al. as reference 8). This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages. About This PDQ SummaryPurpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood central nervous system germ cell tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: - be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Childhood Central Nervous System Germ Cell Tumors Treatment are: - Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Karen J. Marcus, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Roger J. Packer, MD (Children's National Health System)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Central Nervous System Germ Cell Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-cns-germ-cell-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389498] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2016-09-23 Last modified on: 8 September 2017
|
|